

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50049707 (6R,7R)-7-{2-(2-Amino-thiazol-4-yl)-2-[(Z)-methoxyimino]-acetylamino}-3-(2-methyl-5,6-dioxo-1,2,5,6-tetrahydro-[1,2,4]triazin-3-ylsulfanylmethyl)-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid::(6R,7R)-7-{2-(2-Amino-thiazol-4-yl)-2-[(Z)-methoxyimino]-acetylamino}-3-(6-hydroxy-2-methyl-5-oxo-2,5-dihydro-[1,2,4]triazin-3-ylsulfanylmethyl)-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid::(6R,7R,)-7-(2-(2-aminothiazol-4-yl)-2-(methoxyimino)acetamido)-3-((2-methyl-5,6-dioxo-1,2,5,6-tetrahydro-1,2,4-triazin-3-ylthio)methyl)-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid::(6R,7R,Z)-7-(2-(2-aminothiazol-4-yl)-2-(methoxyimino)acetamido)-3-((2-methyl-5,6-dioxo-1,2,5,6-tetrahydro-1,2,4-triazin-3-ylthio)methyl)-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid::7-[2-(2-Amino-thiazol-4-yl)-2-methoxyimino-acetylamino]-3-(6-hydroxy-2-methyl-5-oxo-2,5-dihydro-[1,2,4]triazin-3-ylsulfanylmethyl)-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid (Ceftriaxone)::7-[2-(2-Amino-thiazol-4-yl)-2-methoxyimino-acetylamino]-3-(6-hydroxy-2-methyl-5-oxo-2,5-dihydro-[1,2,4]triazin-3-ylsulfanylmethyl)-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid(Ceftriaxone)::CHEMBL161::ceftriaxone
SMILES: CO\N=C(/C(=O)N[C@H]1[C@H]2SCC(CSc3nc(=O)c(=O)[nH]n3C)=C(N2C1=O)C(O)=O)c1csc(N)n1
InChI Key: InChIKey=VAAUVRVFOQPIGI-SPQHTLEESA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Solute carrier family 22 member 6 (Homo sapiens (Human)) | BDBM50049707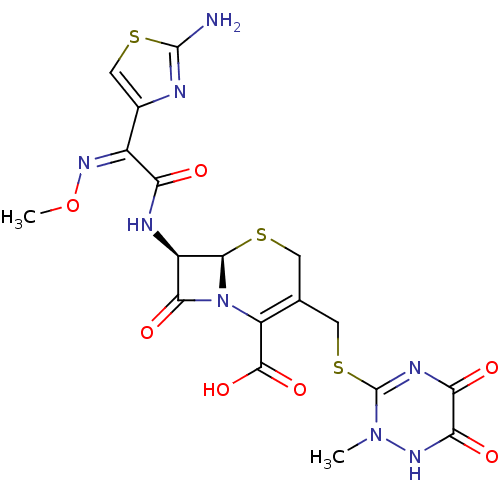 ((6R,7R)-7-{2-(2-Amino-thiazol-4-yl)-2-[(Z)-methoxy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of PAH uptake in OAT1-expressing S2 cells | Eur J Pharmacol 438: 137-42 (2002) BindingDB Entry DOI: 10.7270/Q2N87C2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 11 (Homo sapiens (Human)) | BDBM50049707 ((6R,7R)-7-{2-(2-Amino-thiazol-4-yl)-2-[(Z)-methoxy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | 2.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Estrone sulfate uptake in OAT4-expressing S2 cells | Eur J Pharmacol 438: 137-42 (2002) BindingDB Entry DOI: 10.7270/Q2N87C2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 8 (Homo sapiens (Human)) | BDBM50049707 ((6R,7R)-7-{2-(2-Amino-thiazol-4-yl)-2-[(Z)-methoxy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | 4.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin University School of Medicine Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Estrone sulfate uptake in OAT3-expressing S2 cells | Eur J Pharmacol 438: 137-42 (2002) BindingDB Entry DOI: 10.7270/Q2N87C2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50049707 ((6R,7R)-7-{2-(2-Amino-thiazol-4-yl)-2-[(Z)-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.58E+4 | -6.55 | 2.10E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Erzincan University | Assay Description CA activity was assayed according to method of Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] described previously by Innocenti e... | J Enzyme Inhib Med Chem 27: 641-5 (2012) Article DOI: 10.3109/14756366.2011.604852 BindingDB Entry DOI: 10.7270/Q2WH2NXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione reductase (GR) (Homo sapiens (Human)) | BDBM50049707 ((6R,7R)-7-{2-(2-Amino-thiazol-4-yl)-2-[(Z)-methoxy...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.73E+4 | n/a | 2.13E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Ondokuz Mayis University | Assay Description Enzymatic activity was measured by Beutler's method with a Shimadzu Spectrophotometer UV-(1208), at 25°C. The assay system contained 100 mM Tris-... | J Enzyme Inhib Med Chem 28: 824-9 (2013) Article DOI: 10.3109/14756366.2012.688042 BindingDB Entry DOI: 10.7270/Q21J98P4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50049707 ((6R,7R)-7-{2-(2-Amino-thiazol-4-yl)-2-[(Z)-methoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.85E+4 | -6.20 | 2.71E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Erzincan University | Assay Description CA activity was assayed according to method of Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] described previously by Innocenti e... | J Enzyme Inhib Med Chem 27: 641-5 (2012) Article DOI: 10.3109/14756366.2011.604852 BindingDB Entry DOI: 10.7270/Q2WH2NXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione peroxidase 1 (GPx) (Homo sapiens (Human)) | BDBM50049707 ((6R,7R)-7-{2-(2-Amino-thiazol-4-yl)-2-[(Z)-methoxy...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 3.41E+4 | n/a | 4.86E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Ondokuz Mayis University | Assay Description Glutathione peroxidase was assayed in a l-ml system containing 0.1 M potassium phosphate buffer, pH 7.0, 0.2 mM NADPH, 1 i.u. glutathione reductase, ... | J Enzyme Inhib Med Chem 28: 824-9 (2013) Article DOI: 10.3109/14756366.2012.688042 BindingDB Entry DOI: 10.7270/Q21J98P4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase (GST) (Homo sapiens (Human)) | BDBM50049707 ((6R,7R)-7-{2-(2-Amino-thiazol-4-yl)-2-[(Z)-methoxy...) | UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 3.54E+4 | n/a | 6.18E+4 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ondokuz Mayis University | Assay Description Enzymatic activity was determined spectrophotometrically by measuring the conjugation of CDNB with GSH. The 1 ml assay mixture contained 0.5 mM CDNB,... | J Enzyme Inhib Med Chem 28: 824-9 (2013) Article DOI: 10.3109/14756366.2012.688042 BindingDB Entry DOI: 10.7270/Q21J98P4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligopeptide transporter, kidney isoform (Rattus norvegicus) | BDBM50049707 ((6R,7R)-7-{2-(2-Amino-thiazol-4-yl)-2-[(Z)-methoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biozentrum of the Martin-Luther-University Halle-Wittenberg Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Gly-Sar uptake (pH6.0) in SKPT cells | Eur J Pharm Biopharm 59: 17-24 (2004) Article DOI: 10.1016/j.ejpb.2004.07.008 BindingDB Entry DOI: 10.7270/Q2TT4S7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligopeptide transporter small intestine isoform (Homo sapiens (Human)) | BDBM50049707 ((6R,7R)-7-{2-(2-Amino-thiazol-4-yl)-2-[(Z)-methoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | >3.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-University Halle-Wittenberg Curated by ChEMBL | Assay Description Binding affinity against membrane transport protein PEPT1 in human Caco-2 cells | J Med Chem 48: 4410-9 (2005) Article DOI: 10.1021/jm048982w BindingDB Entry DOI: 10.7270/Q2Q24116 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligopeptide transporter small intestine isoform (Homo sapiens (Human)) | BDBM50049707 ((6R,7R)-7-{2-(2-Amino-thiazol-4-yl)-2-[(Z)-methoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | >3.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biozentrum of the Martin-Luther-University Halle-Wittenberg Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Gly-Sar uptake (pH6.0) in Caco-2 cells | Eur J Pharm Biopharm 59: 17-24 (2004) Article DOI: 10.1016/j.ejpb.2004.07.008 BindingDB Entry DOI: 10.7270/Q2TT4S7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 4 (Homo sapiens (Human)) | BDBM50049707 ((6R,7R)-7-{2-(2-Amino-thiazol-4-yl)-2-[(Z)-methoxy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Canalicular multispecific organic anion transporter 1 (Homo sapiens (Human)) | BDBM50049707 ((6R,7R)-7-{2-(2-Amino-thiazol-4-yl)-2-[(Z)-methoxy...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP2 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Canalicular multispecific organic anion transporter 2 (Homo sapiens (Human)) | BDBM50049707 ((6R,7R)-7-{2-(2-Amino-thiazol-4-yl)-2-[(Z)-methoxy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human MRP3 overexpressed in Sf9 insect cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile salt export pump (Homo sapiens (Human)) | BDBM50049707 ((6R,7R)-7-{2-(2-Amino-thiazol-4-yl)-2-[(Z)-methoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc Curated by ChEMBL | Assay Description Inhibition of human BSEP overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-taurocholate in presence of ATP measured after 15 to ... | Toxicol Sci 136: 216-41 (2013) BindingDB Entry DOI: 10.7270/Q2JM2D2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||