 Found 11 hits for monomerid = 50049820
Found 11 hits for monomerid = 50049820 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Rhodopsin kinase
(Homo sapiens (Human)) | BDBM50049820
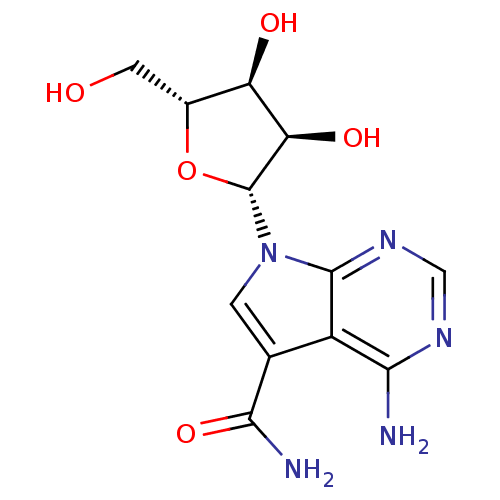
(4-Amino-5-carboxamide-7-(D-ribofuranosyl)pyrrolo[2...)Show SMILES NC(=O)c1cn([C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)c2ncnc(N)c12 |r| Show InChI InChI=1S/C12H15N5O5/c13-9-6-4(10(14)21)1-17(11(6)16-3-15-9)12-8(20)7(19)5(2-18)22-12/h1,3,5,7-8,12,18-20H,2H2,(H2,14,21)(H2,13,15,16)/t5-,7-,8-,12-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of rhodopsin kinase (unknown origin) |
J Biol Chem 282: 15271-83 (2007)
Article DOI: 10.1074/jbc.M701362200
BindingDB Entry DOI: 10.7270/Q2XD11FR |
More data for this
Ligand-Target Pair | |
Beta-adrenergic receptor kinase 1
(Homo sapiens (Human)) | BDBM50049820

(4-Amino-5-carboxamide-7-(D-ribofuranosyl)pyrrolo[2...)Show SMILES NC(=O)c1cn([C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)c2ncnc(N)c12 |r| Show InChI InChI=1S/C12H15N5O5/c13-9-6-4(10(14)21)1-17(11(6)16-3-15-9)12-8(20)7(19)5(2-18)22-12/h1,3,5,7-8,12,18-20H,2H2,(H2,14,21)(H2,13,15,16)/t5-,7-,8-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of beta-adrenergic receptor kinase(unknown origin) |
J Biol Chem 282: 15271-83 (2007)
Article DOI: 10.1074/jbc.M701362200
BindingDB Entry DOI: 10.7270/Q2XD11FR |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50049820

(4-Amino-5-carboxamide-7-(D-ribofuranosyl)pyrrolo[2...)Show SMILES NC(=O)c1cn([C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)c2ncnc(N)c12 |r| Show InChI InChI=1S/C12H15N5O5/c13-9-6-4(10(14)21)1-17(11(6)16-3-15-9)12-8(20)7(19)5(2-18)22-12/h1,3,5,7-8,12,18-20H,2H2,(H2,14,21)(H2,13,15,16)/t5-,7-,8-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human adenosine kinase |
J Med Chem 43: 2883-93 (2000)
BindingDB Entry DOI: 10.7270/Q2XG9QCV |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50049820

(4-Amino-5-carboxamide-7-(D-ribofuranosyl)pyrrolo[2...)Show SMILES NC(=O)c1cn([C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)c2ncnc(N)c12 |r| Show InChI InChI=1S/C12H15N5O5/c13-9-6-4(10(14)21)1-17(11(6)16-3-15-9)12-8(20)7(19)5(2-18)22-12/h1,3,5,7-8,12,18-20H,2H2,(H2,14,21)(H2,13,15,16)/t5-,7-,8-,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of DOT1L (unknown origin) using chicken nucleosome as substrate in presence of [3H]SAM incubated for 1 hr by TopCount method |
Bioorg Med Chem Lett 26: 4518-4522 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.041 |
More data for this
Ligand-Target Pair | |
heat shock 70kDa protein 8 isoform 1
(Homo sapiens (Human)) | BDBM50049820

(4-Amino-5-carboxamide-7-(D-ribofuranosyl)pyrrolo[2...)Show SMILES NC(=O)c1cn([C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)c2ncnc(N)c12 |r| Show InChI InChI=1S/C12H15N5O5/c13-9-6-4(10(14)21)1-17(11(6)16-3-15-9)12-8(20)7(19)5(2-18)22-12/h1,3,5,7-8,12,18-20H,2H2,(H2,14,21)(H2,13,15,16)/t5-,7-,8-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Binding affinity to human truncated HSC70 NBD (1 to 381 residues) by SPR analysis |
J Med Chem 59: 4625-36 (2016)
BindingDB Entry DOI: 10.7270/Q2Z03B3X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50049820

(4-Amino-5-carboxamide-7-(D-ribofuranosyl)pyrrolo[2...)Show SMILES NC(=O)c1cn([C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)c2ncnc(N)c12 |r| Show InChI InChI=1S/C12H15N5O5/c13-9-6-4(10(14)21)1-17(11(6)16-3-15-9)12-8(20)7(19)5(2-18)22-12/h1,3,5,7-8,12,18-20H,2H2,(H2,14,21)(H2,13,15,16)/t5-,7-,8-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 467 | n/a | n/a | n/a | n/a | n/a | n/a |
Vigo University
Curated by ChEMBL
| Assay Description
Concentration required for 50% inhibition of the adenosine kinase (AK) activity. |
Bioorg Med Chem Lett 14: 3077-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.040
BindingDB Entry DOI: 10.7270/Q2CZ38BM |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50049820

(4-Amino-5-carboxamide-7-(D-ribofuranosyl)pyrrolo[2...)Show SMILES NC(=O)c1cn([C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)c2ncnc(N)c12 |r| Show InChI InChI=1S/C12H15N5O5/c13-9-6-4(10(14)21)1-17(11(6)16-3-15-9)12-8(20)7(19)5(2-18)22-12/h1,3,5,7-8,12,18-20H,2H2,(H2,14,21)(H2,13,15,16)/t5-,7-,8-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.70E+8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of adenosine kinase (unknown origin) |
Citation and Details
Article DOI: 10.1007/s00044-004-0048-0
BindingDB Entry DOI: 10.7270/Q2XG9V11 |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50049820

(4-Amino-5-carboxamide-7-(D-ribofuranosyl)pyrrolo[2...)Show SMILES NC(=O)c1cn([C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)c2ncnc(N)c12 |r| Show InChI InChI=1S/C12H15N5O5/c13-9-6-4(10(14)21)1-17(11(6)16-3-15-9)12-8(20)7(19)5(2-18)22-12/h1,3,5,7-8,12,18-20H,2H2,(H2,14,21)(H2,13,15,16)/t5-,7-,8-,12-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University
Curated by ChEMBL
| Assay Description
Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... |
J Med Chem 62: 4483-4499 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00020 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50049820

(4-Amino-5-carboxamide-7-(D-ribofuranosyl)pyrrolo[2...)Show SMILES NC(=O)c1cn([C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)c2ncnc(N)c12 |r| Show InChI InChI=1S/C12H15N5O5/c13-9-6-4(10(14)21)1-17(11(6)16-3-15-9)12-8(20)7(19)5(2-18)22-12/h1,3,5,7-8,12,18-20H,2H2,(H2,14,21)(H2,13,15,16)/t5-,7-,8-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of human adenosine kinase activity |
Bioorg Med Chem Lett 12: 899-902 (2002)
BindingDB Entry DOI: 10.7270/Q2NP23QT |
More data for this
Ligand-Target Pair | |
Galanin receptor 3
(Homo sapiens (Human)) | BDBM50049820

(4-Amino-5-carboxamide-7-(D-ribofuranosyl)pyrrolo[2...)Show SMILES NC(=O)c1cn([C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)c2ncnc(N)c12 |r| Show InChI InChI=1S/C12H15N5O5/c13-9-6-4(10(14)21)1-17(11(6)16-3-15-9)12-8(20)7(19)5(2-18)22-12/h1,3,5,7-8,12,18-20H,2H2,(H2,14,21)(H2,13,15,16)/t5-,7-,8-,12-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
| |
PubChem Bioassay (2013)
BindingDB Entry DOI: 10.7270/Q270802Q |
More data for this
Ligand-Target Pair | |
heat shock 70kDa protein 8 isoform 1
(Homo sapiens (Human)) | BDBM50049820

(4-Amino-5-carboxamide-7-(D-ribofuranosyl)pyrrolo[2...)Show SMILES NC(=O)c1cn([C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)c2ncnc(N)c12 |r| Show InChI InChI=1S/C12H15N5O5/c13-9-6-4(10(14)21)1-17(11(6)16-3-15-9)12-8(20)7(19)5(2-18)22-12/h1,3,5,7-8,12,18-20H,2H2,(H2,14,21)(H2,13,15,16)/t5-,7-,8-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | n/a | 3.24E+3 | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Binding affinity to human truncated HSC70 NBD (1 to 381 residues) by SPR analysis |
J Med Chem 59: 4625-36 (2016)
BindingDB Entry DOI: 10.7270/Q2Z03B3X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data