 Found 5 hits for monomerid = 50050712
Found 5 hits for monomerid = 50050712 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50050712
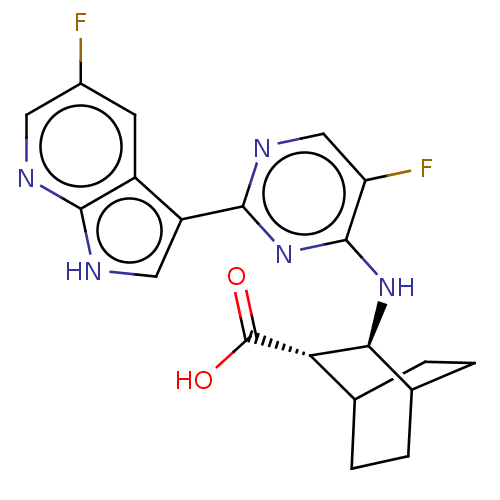
(CHEMBL3318007)Show SMILES OC(=O)[C@H]1C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wU:3.2,wD:10.12,(15.61,-25.66,;14.11,-26.02,;13.05,-24.9,;13.67,-27.49,;14.73,-28.62,;14.29,-30.09,;12.8,-30.45,;11.74,-29.34,;12.83,-28.23,;13.22,-29.72,;12.18,-27.86,;11.12,-26.75,;9.63,-27.1,;9.19,-28.58,;7.69,-28.94,;6.63,-27.82,;7.06,-26.35,;8.56,-25.99,;9,-24.51,;7.25,-30.41,;8.2,-31.63,;7.32,-32.91,;5.84,-32.47,;4.53,-33.27,;3.18,-32.54,;3.14,-31,;1.79,-30.27,;4.45,-30.19,;5.8,-30.93,)| Show InChI InChI=1S/C20H19F2N5O2/c21-11-5-12-13(7-24-17(12)23-6-11)18-25-8-14(22)19(27-18)26-16-10-3-1-9(2-4-10)15(16)20(28)29/h5-10,15-16H,1-4H2,(H,23,24)(H,28,29)(H,25,26,27)/t9?,10?,15-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) |
J Med Chem 57: 6668-78 (2014)
Article DOI: 10.1021/jm5007275
BindingDB Entry DOI: 10.7270/Q2R21316 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50050712

(CHEMBL3318007)Show SMILES OC(=O)[C@H]1C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wU:3.2,wD:10.12,(15.61,-25.66,;14.11,-26.02,;13.05,-24.9,;13.67,-27.49,;14.73,-28.62,;14.29,-30.09,;12.8,-30.45,;11.74,-29.34,;12.83,-28.23,;13.22,-29.72,;12.18,-27.86,;11.12,-26.75,;9.63,-27.1,;9.19,-28.58,;7.69,-28.94,;6.63,-27.82,;7.06,-26.35,;8.56,-25.99,;9,-24.51,;7.25,-30.41,;8.2,-31.63,;7.32,-32.91,;5.84,-32.47,;4.53,-33.27,;3.18,-32.54,;3.14,-31,;1.79,-30.27,;4.45,-30.19,;5.8,-30.93,)| Show InChI InChI=1S/C20H19F2N5O2/c21-11-5-12-13(7-24-17(12)23-6-11)18-25-8-14(22)19(27-18)26-16-10-3-1-9(2-4-10)15(16)20(28)29/h5-10,15-16H,1-4H2,(H,23,24)(H,28,29)(H,25,26,27)/t9?,10?,15-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3 (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50050712

(CHEMBL3318007)Show SMILES OC(=O)[C@H]1C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wU:3.2,wD:10.12,(15.61,-25.66,;14.11,-26.02,;13.05,-24.9,;13.67,-27.49,;14.73,-28.62,;14.29,-30.09,;12.8,-30.45,;11.74,-29.34,;12.83,-28.23,;13.22,-29.72,;12.18,-27.86,;11.12,-26.75,;9.63,-27.1,;9.19,-28.58,;7.69,-28.94,;6.63,-27.82,;7.06,-26.35,;8.56,-25.99,;9,-24.51,;7.25,-30.41,;8.2,-31.63,;7.32,-32.91,;5.84,-32.47,;4.53,-33.27,;3.18,-32.54,;3.14,-31,;1.79,-30.27,;4.45,-30.19,;5.8,-30.93,)| Show InChI InChI=1S/C20H19F2N5O2/c21-11-5-12-13(7-24-17(12)23-6-11)18-25-8-14(22)19(27-18)26-16-10-3-1-9(2-4-10)15(16)20(28)29/h5-10,15-16H,1-4H2,(H,23,24)(H,28,29)(H,25,26,27)/t9?,10?,15-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50050712

(CHEMBL3318007)Show SMILES OC(=O)[C@H]1C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wU:3.2,wD:10.12,(15.61,-25.66,;14.11,-26.02,;13.05,-24.9,;13.67,-27.49,;14.73,-28.62,;14.29,-30.09,;12.8,-30.45,;11.74,-29.34,;12.83,-28.23,;13.22,-29.72,;12.18,-27.86,;11.12,-26.75,;9.63,-27.1,;9.19,-28.58,;7.69,-28.94,;6.63,-27.82,;7.06,-26.35,;8.56,-25.99,;9,-24.51,;7.25,-30.41,;8.2,-31.63,;7.32,-32.91,;5.84,-32.47,;4.53,-33.27,;3.18,-32.54,;3.14,-31,;1.79,-30.27,;4.45,-30.19,;5.8,-30.93,)| Show InChI InChI=1S/C20H19F2N5O2/c21-11-5-12-13(7-24-17(12)23-6-11)18-25-8-14(22)19(27-18)26-16-10-3-1-9(2-4-10)15(16)20(28)29/h5-10,15-16H,1-4H2,(H,23,24)(H,28,29)(H,25,26,27)/t9?,10?,15-,16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) |
Bioorg Med Chem Lett 25: 1990-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.013
BindingDB Entry DOI: 10.7270/Q2V40WW5 |
More data for this
Ligand-Target Pair | |
Polymerase basic protein 2
(Influenza A virus (strain A/Puerto Rico/8/1934 H1N...) | BDBM50050712

(CHEMBL3318007)Show SMILES OC(=O)[C@H]1C2CCC(CC2)[C@@H]1Nc1nc(ncc1F)-c1c[nH]c2ncc(F)cc12 |r,wU:3.2,wD:10.12,(15.61,-25.66,;14.11,-26.02,;13.05,-24.9,;13.67,-27.49,;14.73,-28.62,;14.29,-30.09,;12.8,-30.45,;11.74,-29.34,;12.83,-28.23,;13.22,-29.72,;12.18,-27.86,;11.12,-26.75,;9.63,-27.1,;9.19,-28.58,;7.69,-28.94,;6.63,-27.82,;7.06,-26.35,;8.56,-25.99,;9,-24.51,;7.25,-30.41,;8.2,-31.63,;7.32,-32.91,;5.84,-32.47,;4.53,-33.27,;3.18,-32.54,;3.14,-31,;1.79,-30.27,;4.45,-30.19,;5.8,-30.93,)| Show InChI InChI=1S/C20H19F2N5O2/c21-11-5-12-13(7-24-17(12)23-6-11)18-25-8-14(22)19(27-18)26-16-10-3-1-9(2-4-10)15(16)20(28)29/h5-10,15-16H,1-4H2,(H,23,24)(H,28,29)(H,25,26,27)/t9?,10?,15-,16-/m0/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of PB2 in Influenza A virus (A/Weiss/1943(H1N1)) infected in MDCK cells assessed as reduction in virus induced cell death after 5 days by ... |
Eur J Med Chem 162: 249-265 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.015 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data