

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CN1CCCNC(=O)CCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C1=O)C(N)=O
InChI Key: InChIKey=ZFMPRZDNGINSND-ISWDJSIVSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50052522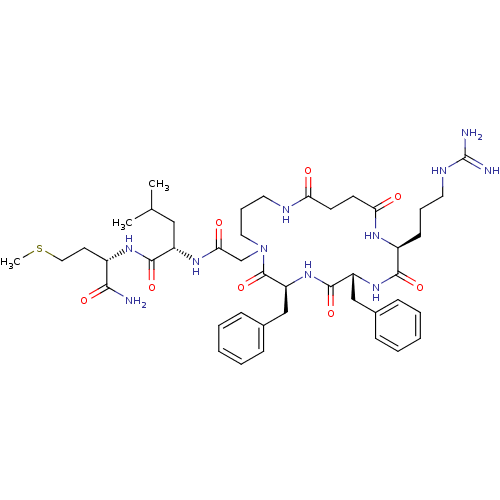 ((S)-2-{2-[(2S,5R,8S)-5,8-Dibenzyl-2-(3-guanidino-p...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 2 in rat vas deferens (RVD) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50052522 ((S)-2-{2-[(2S,5R,8S)-5,8-Dibenzyl-2-(3-guanidino-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 1 in guinea pig ileum(GPI) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Rattus norvegicus) | BDBM50052522 ((S)-2-{2-[(2S,5R,8S)-5,8-Dibenzyl-2-(3-guanidino-p...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 3 in rat portal vein (RPV) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||