

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50052693 (2S,3S)-3-[(S)-3-Methyl-1-(3-methyl-butylcarbamoyl)-butylcarbamoyl]-oxirane-2-carboxylic acid ethyl ester::(2S,3S)-ethyl 3-(((S)-1-(isopentylamino)-4-methyl-1-oxopentan-2-yl)carbamoyl)oxirane-2-carboxylate::(2S,3S)-trans-epoxysuccinyl-L-leucylamido-3-methylbutane ethyl ester::3-[3-Methyl-1-(3-methyl-butylcarbamoyl)-butylcarbamoyl]-oxirane-2-carboxylic acid ethyl ester::Aloxistatin::CHEMBL63440
SMILES: CCOC(=O)[C@H]1O[C@@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCCC(C)C
InChI Key: InChIKey=SRVFFFJZQVENJC-IHRRRGAJSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Papain (Carica papaya) | BDBM50052693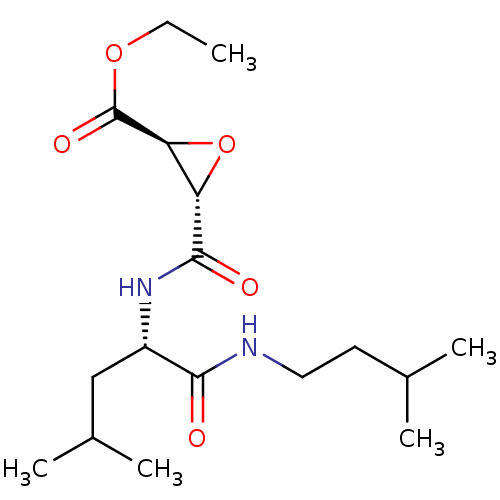 ((2S,3S)-3-[(S)-3-Methyl-1-(3-methyl-butylcarbamoyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Kinetic constant Apparent binding constant (Ki`) for the inhibition of papain conducted in 0.1 M phosphate, pH 6.8, at 30 degree C | J Med Chem 39: 3357-66 (1996) Article DOI: 10.1021/jm950445b BindingDB Entry DOI: 10.7270/Q2B56HTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50052693 ((2S,3S)-3-[(S)-3-Methyl-1-(3-methyl-butylcarbamoyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Blockade of cathepsin G processing in human U937 cells by densitometry | J Biol Chem 282: 20836-46 (2007) Article DOI: 10.1074/jbc.M702615200 BindingDB Entry DOI: 10.7270/Q2125TJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain1 (Homo sapiens (Human)) | BDBM50052693 ((2S,3S)-3-[(S)-3-Methyl-1-(3-methyl-butylcarbamoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Health Science Center Curated by ChEMBL | Assay Description The calpain inhibitory activity(I 50) was measured as ability to enter the platelet to inhibit calpain after cell lysis(assay 2) | J Med Chem 35: 2048-54 (1992) BindingDB Entry DOI: 10.7270/Q27M094H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Mus musculus) | BDBM50052693 ((2S,3S)-3-[(S)-3-Methyl-1-(3-methyl-butylcarbamoyl...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of cathepsin G activation in beta-estradiol differentiated mouse EcoM-G cells after 24 hrs | J Biol Chem 282: 20836-46 (2007) Article DOI: 10.1074/jbc.M702615200 BindingDB Entry DOI: 10.7270/Q2125TJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Mus musculus) | BDBM50052693 ((2S,3S)-3-[(S)-3-Methyl-1-(3-methyl-butylcarbamoyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of proteinase-3 activation in beta-estradiol differentiated mouse EcoM-G cells after 24 hrs | J Biol Chem 282: 20836-46 (2007) Article DOI: 10.1074/jbc.M702615200 BindingDB Entry DOI: 10.7270/Q2125TJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain1 (Homo sapiens (Human)) | BDBM50052693 ((2S,3S)-3-[(S)-3-Methyl-1-(3-methyl-butylcarbamoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Health Science Center Curated by ChEMBL | Assay Description The ability of compound to inhibit calpain in a preparation of lysed platelets was measured with a caseinolytic assay(assay 1) | J Med Chem 35: 2048-54 (1992) BindingDB Entry DOI: 10.7270/Q27M094H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Mus musculus) | BDBM50052693 ((2S,3S)-3-[(S)-3-Methyl-1-(3-methyl-butylcarbamoyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of neutrophil elastase activation in beta-estradiol differentiated mouse EcoM-G cells after 24 hrs | J Biol Chem 282: 20836-46 (2007) Article DOI: 10.1074/jbc.M702615200 BindingDB Entry DOI: 10.7270/Q2125TJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||