

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: [#6]-[#7](-[#6])-c1ccc(cc1)-[#6](=[#6]-1\[#6]=[#6]/[#6](/[#6]=[#6]-1)=[#7+](\[#6])-[#6])\c1ccc(cc1)-[#7](-[#6])-[#6]
InChI Key: InChIKey=LGLFFNDHMLKUMI-UHFFFAOYSA-N
PDB links: 5 PDB IDs match this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Microtubule-associated protein (Homo sapiens (Human)) | BDBM50052802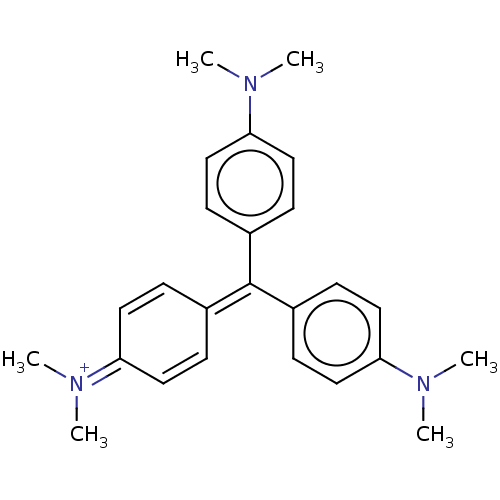 (CHEBI:77181 | CRYSTAL VIOLET | Crystal violet (15)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | 7.4 | n/a |
The Ohio State University | Assay Description The Tau content of fractions was determined by dot blot analysis using nitrocellulose membranes (0.2 μm porosity) as described previously [Cisek... | J Biol Chem 288: 32599-611 (2013) Article DOI: 10.1074/jbc.M113.503474 BindingDB Entry DOI: 10.7270/Q2R49PMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyribonuclease-1 (Homo sapiens (Human)) | BDBM50052802 (CHEBI:77181 | CRYSTAL VIOLET | Crystal violet (15)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nis Curated by ChEMBL | Assay Description Inhibition of DNase 1 (unknown origin) using (FAM)-labeled dsDNA as substrate | Eur J Med Chem 88: 101-11 (2014) Article DOI: 10.1016/j.ejmech.2014.07.040 BindingDB Entry DOI: 10.7270/Q2M32XC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Co-chaperonin GroES (Escherichia coli) | BDBM50052802 (CHEBI:77181 | CRYSTAL VIOLET | Crystal violet (15)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GroEL expressed in Escherichia coliDH5alpha/Escherichia coli GroES expressed in Escherichia coli BL21 (DE3) assessed a... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Co-chaperonin GroES (Escherichia coli) | BDBM50052802 (CHEBI:77181 | CRYSTAL VIOLET | Crystal violet (15)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GroEL expressed in Escherichia coli DH5alpha/Escherichia coli GroES expressed in Escherichia coli BL21 (DE3) assessed ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chaperonin GroEL (Escherichia coli) | BDBM50052802 (CHEBI:77181 | CRYSTAL VIOLET | Crystal violet (15)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ATPase activity of Escherichia coli GroEL expressed in Escherichia coliDH5alpha incubated for 60 mins using ATP by spectrometric analys... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50052802 (CHEBI:77181 | CRYSTAL VIOLET | Crystal violet (15)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of native rhodanese (unknown origin) assessed as reduction in rhodanese enzyme activity after 45 mins by Fe(SCN)3 dye based spectrometric ... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 60 kDa heat shock protein, mitochondrial (Homo sapiens) | BDBM50052802 (CHEBI:77181 | CRYSTAL VIOLET | Crystal violet (15)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human N-terminal octa-His-tagged HSP60 expressed in Escherichia coli Rosetta(DE3) pLysS/human HSP10 expressed in Escherichia coli Roset... | Bioorg Med Chem Lett 29: 1106-1112 (2019) Article DOI: 10.1016/j.bmcl.2019.02.028 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||