

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50053179 CHEMBL121924::Sodium; (2S,3S,5R)-3-methyl-4,4,7-trioxo-3-((Z)-2-pyridin-2-yl-vinyl)-4lambda*6*-thia-1-aza-bicyclo[3.2.0]heptane-2-carboxylate
SMILES: C[C@]1(\C=C/c2ccccn2)[C@@H](N2[C@@H](CC2=O)S1(=O)=O)C([O-])=O
InChI Key: InChIKey=ZWKSSRXNDMXRII-KGKKKFOGSA-M
Data: 12 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Penicillin-binding protein (Staphylococcus aureus) | BDBM50053179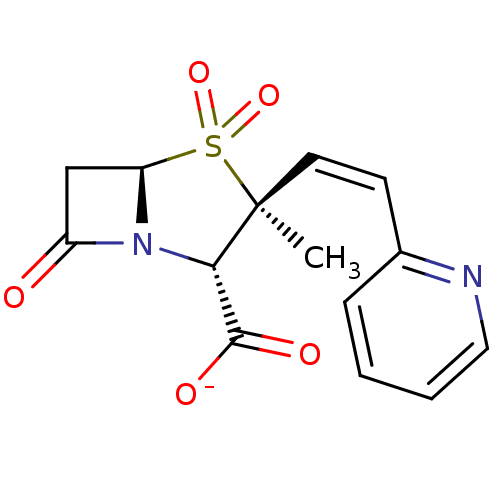 (CHEMBL121924 | Sodium; (2S,3S,5R)-3-methyl-4,4,7-t...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Penicillin-binding protein 2 from Staphylococcus aureus (Schoch). | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillin-binding protein (Staphylococcus aureus) | BDBM50053179 (CHEMBL121924 | Sodium; (2S,3S,5R)-3-methyl-4,4,7-t...) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Penicillin-binding protein 3 from Escherichia coli. | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillin-binding protein (Staphylococcus aureus) | BDBM50053179 (CHEMBL121924 | Sodium; (2S,3S,5R)-3-methyl-4,4,7-t...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Penicillin-binding protein 2 from Escherichia coli. | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillin-binding protein (Staphylococcus aureus) | BDBM50053179 (CHEMBL121924 | Sodium; (2S,3S,5R)-3-methyl-4,4,7-t...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of 604 Cephalosporin resistant mutants of Penicillin-binding protein 2 from Streptococcus pneumoniae | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50053179 (CHEMBL121924 | Sodium; (2S,3S,5R)-3-methyl-4,4,7-t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Pseudomonas aeruginosa 18SH ,class C of Beta-lactamase | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillin-binding protein (Staphylococcus aureus) | BDBM50053179 (CHEMBL121924 | Sodium; (2S,3S,5R)-3-methyl-4,4,7-t...) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Penicillin-binding protein 3 from Escherichia coli. | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillin-binding protein (Staphylococcus aureus) | BDBM50053179 (CHEMBL121924 | Sodium; (2S,3S,5R)-3-methyl-4,4,7-t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Penicillin-binding protein 4 from Staphylococcus aureus (Schoch). | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Bacillus licheniformis) | BDBM50053179 (CHEMBL121924 | Sodium; (2S,3S,5R)-3-methyl-4,4,7-t...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Bacillus licheniformis 749/C ,class A of Beta-lactamase | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Citrobacter freundii) | BDBM50053179 (CHEMBL121924 | Sodium; (2S,3S,5R)-3-methyl-4,4,7-t...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Citrobacter freundii 1928, class C of Beta-lactamase | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillin-binding protein (Staphylococcus aureus) | BDBM50053179 (CHEMBL121924 | Sodium; (2S,3S,5R)-3-methyl-4,4,7-t...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of 503 Cephalosporin resistant mutants of Penicillin-binding protein 2 from Streptococcus pneumoniae | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bacterial penicillin-binding protein (Escherichia coli (strain K12)) | BDBM50053179 (CHEMBL121924 | Sodium; (2S,3S,5R)-3-methyl-4,4,7-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Penicillin-binding protein 1b from Escherichia coli. | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase AmpC (Escherichia coli) | BDBM50053179 (CHEMBL121924 | Sodium; (2S,3S,5R)-3-methyl-4,4,7-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Escherichia coli AmpC ,class C of Beta-lactamase | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||