 Found 12 hits for monomerid = 50053435
Found 12 hits for monomerid = 50053435 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50053435
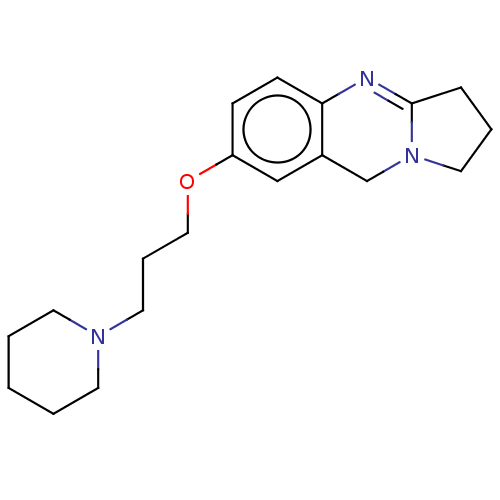
(CHEMBL3323035)Show InChI InChI=1S/C19H27N3O/c1-2-9-21(10-3-1)11-5-13-23-17-7-8-18-16(14-17)15-22-12-4-6-19(22)20-18/h7-8,14H,1-6,9-13,15H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H3 receptor expressed in Sf9 insect cell membranes after 60 mins by liquid scintillation counting ... |
Bioorg Med Chem Lett 26: 4140-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.054 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50053435

(CHEMBL3323035)Show InChI InChI=1S/C19H27N3O/c1-2-9-21(10-3-1)11-5-13-23-17-7-8-18-16(14-17)15-22-12-4-6-19(22)20-18/h7-8,14H,1-6,9-13,15H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H3 receptor expressed in Sf9 insect cell membranes after 60 mins by liquid scintillation counting ... |
Bioorg Med Chem Lett 26: 4140-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.054 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50053435

(CHEMBL3323035)Show InChI InChI=1S/C19H27N3O/c1-2-9-21(10-3-1)11-5-13-23-17-7-8-18-16(14-17)15-22-12-4-6-19(22)20-18/h7-8,14H,1-6,9-13,15H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Eur J Med Chem 180: 690-706 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.040 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50053435

(CHEMBL3323035)Show InChI InChI=1S/C19H27N3O/c1-2-9-21(10-3-1)11-5-13-23-17-7-8-18-16(14-17)15-22-12-4-6-19(22)20-18/h7-8,14H,1-6,9-13,15H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of human H3 receptor |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053435

(CHEMBL3323035)Show InChI InChI=1S/C19H27N3O/c1-2-9-21(10-3-1)11-5-13-23-17-7-8-18-16(14-17)15-22-12-4-6-19(22)20-18/h7-8,14H,1-6,9-13,15H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 4.5 mins followed by substrate addition measured after 2... |
Bioorg Med Chem Lett 26: 4140-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.054 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50053435

(CHEMBL3323035)Show InChI InChI=1S/C19H27N3O/c1-2-9-21(10-3-1)11-5-13-23-17-7-8-18-16(14-17)15-22-12-4-6-19(22)20-18/h7-8,14H,1-6,9-13,15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BChE using butyrylthiocholine chloride as substrate preincubated for 4.5 mins followed by substrate addition measured afte... |
Bioorg Med Chem Lett 26: 4140-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.054 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053435

(CHEMBL3323035)Show InChI InChI=1S/C19H27N3O/c1-2-9-21(10-3-1)11-5-13-23-17-7-8-18-16(14-17)15-22-12-4-6-19(22)20-18/h7-8,14H,1-6,9-13,15H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 4.5 mins followed by substrate addition measured after 2... |
Bioorg Med Chem Lett 26: 4140-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.054 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50053435

(CHEMBL3323035)Show InChI InChI=1S/C19H27N3O/c1-2-9-21(10-3-1)11-5-13-23-17-7-8-18-16(14-17)15-22-12-4-6-19(22)20-18/h7-8,14H,1-6,9-13,15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BChE using butyrylthiocholine chloride as substrate preincubated for 4.5 mins followed by substrate addition measured afte... |
Bioorg Med Chem Lett 26: 4140-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.054 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50053435

(CHEMBL3323035)Show InChI InChI=1S/C19H27N3O/c1-2-9-21(10-3-1)11-5-13-23-17-7-8-18-16(14-17)15-22-12-4-6-19(22)20-18/h7-8,14H,1-6,9-13,15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg
Curated by ChEMBL
| Assay Description
Inhibition of equine BChE |
Eur J Med Chem 180: 690-706 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.040 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50053435

(CHEMBL3323035)Show InChI InChI=1S/C19H27N3O/c1-2-9-21(10-3-1)11-5-13-23-17-7-8-18-16(14-17)15-22-12-4-6-19(22)20-18/h7-8,14H,1-6,9-13,15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of human AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50053435

(CHEMBL3323035)Show InChI InChI=1S/C19H27N3O/c1-2-9-21(10-3-1)11-5-13-23-17-7-8-18-16(14-17)15-22-12-4-6-19(22)20-18/h7-8,14H,1-6,9-13,15H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50053435

(CHEMBL3323035)Show InChI InChI=1S/C19H27N3O/c1-2-9-21(10-3-1)11-5-13-23-17-7-8-18-16(14-17)15-22-12-4-6-19(22)20-18/h7-8,14H,1-6,9-13,15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg
Curated by ChEMBL
| Assay Description
Inhibition of human AChE |
Eur J Med Chem 180: 690-706 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.040 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data