

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50053608 1N-amino(immino)methyl-3-chloroaniline::CHEMBL13823::N-(3-Chloro-phenyl)-guanidine::N-(3-chlorophenyl)guanidine
SMILES: [#7]\[#6](-[#7])=[#7]\c1cccc(Cl)c1
InChI Key: InChIKey=DWLMIHRZURMFAQ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50053608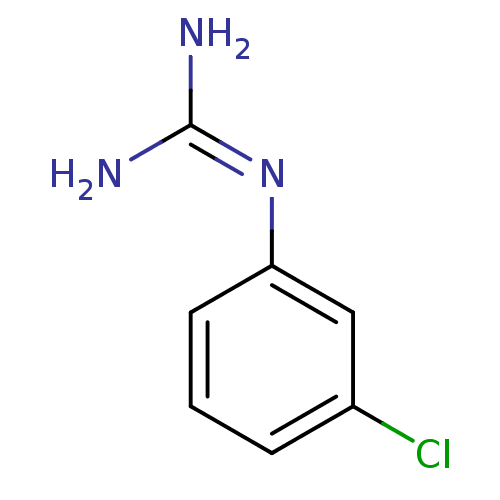 (1N-amino(immino)methyl-3-chloroaniline | CHEMBL138...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]GR65630 from 5-HT3 receptor (unknown origin) expressed in mouse/rat NG108-15 cells after 30 mins by by liquid scintillation count... | Bioorg Med Chem Lett 23: 5945-8 (2013) Article DOI: 10.1016/j.bmcl.2013.08.072 BindingDB Entry DOI: 10.7270/Q2ZK5J31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 3a (5-HT3a)/3b (5-HT3b)/3c (5-HT3c)/3d (5-HT3d)/3e (5-HT3e) receptor (Homo sapiens (Human)) | BDBM50053608 (1N-amino(immino)methyl-3-chloroaniline | CHEMBL138...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Compound was tested for the inhibition of [3H]-GR-65,630 binding to 5-hydroxytryptamine 3 receptor expressed in NG 108-15 cells | J Med Chem 39: 4017-26 (1996) Article DOI: 10.1021/jm9603936 BindingDB Entry DOI: 10.7270/Q23F4NQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator/surface receptor (Homo sapiens (Human)) | BDBM50053608 (1N-amino(immino)methyl-3-chloroaniline | CHEMBL138...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description log1/Ki value was calculated against Urokinase-type plasminogen activator | J Med Chem 33: 2956-61 (1990) BindingDB Entry DOI: 10.7270/Q2GB24NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50053608 (1N-amino(immino)methyl-3-chloroaniline | CHEMBL138...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition against Urokinase-type plasminogen activator | J Med Chem 33: 2956-61 (1990) BindingDB Entry DOI: 10.7270/Q2GB24NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Bos taurus (bovine)) | BDBM50053608 (1N-amino(immino)methyl-3-chloroaniline | CHEMBL138...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition against Trypsin | J Med Chem 33: 2956-61 (1990) BindingDB Entry DOI: 10.7270/Q2GB24NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin I (Bos taurus (bovine)) | BDBM50053608 (1N-amino(immino)methyl-3-chloroaniline | CHEMBL138...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition against Trypsin | J Med Chem 33: 2956-61 (1990) BindingDB Entry DOI: 10.7270/Q2GB24NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50053608 (1N-amino(immino)methyl-3-chloroaniline | CHEMBL138...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | <1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition against human plasmin was determined at 0.5 mM | J Med Chem 33: 2956-61 (1990) BindingDB Entry DOI: 10.7270/Q2GB24NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 1 (Homo sapiens (Human)) | BDBM50053608 (1N-amino(immino)methyl-3-chloroaniline | CHEMBL138...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutics, School of Pharmacy, Virginia Commonwealth University, Richmond, VA 23298, USA. Curated by ChEMBL | Assay Description Inhibition of human OCT1 expressed in HEK293 cells assessed as decrease in uptake of substrate [3H]MPP+ after 1 min by liquid scintillation counting ... | Bioorg Med Chem Lett 27: 4440-4445 (2017) Article DOI: 10.1016/j.bmcl.2017.08.008 BindingDB Entry DOI: 10.7270/Q24M970C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 3 (Homo sapiens (Human)) | BDBM50053608 (1N-amino(immino)methyl-3-chloroaniline | CHEMBL138...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutics, School of Pharmacy, Virginia Commonwealth University, Richmond, VA 23298, USA. Curated by ChEMBL | Assay Description Inhibition of OCT3 (unknown origin) | Bioorg Med Chem Lett 27: 4440-4445 (2017) Article DOI: 10.1016/j.bmcl.2017.08.008 BindingDB Entry DOI: 10.7270/Q24M970C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 2 (Homo sapiens (Human)) | BDBM50053608 (1N-amino(immino)methyl-3-chloroaniline | CHEMBL138...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutics, School of Pharmacy, Virginia Commonwealth University, Richmond, VA 23298, USA. Curated by ChEMBL | Assay Description Inhibition of human OCT2 expressed in HEK293 cells assessed as decrease in uptake of substrate [3H]MPP+ after 1 min by liquid scintillation counting ... | Bioorg Med Chem Lett 27: 4440-4445 (2017) Article DOI: 10.1016/j.bmcl.2017.08.008 BindingDB Entry DOI: 10.7270/Q24M970C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||