

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50054170 2-{3-[4,7-dibenzyl-5,6-dihydroxy-2-oxo-3-[3-(5-trifluoromethyl-1,3,4-thiadiazol-2-ylcarbamoyl)benzyl]-(4R,5S,6S,7R)-1,3-diazepan-1-ylmethyl]phenylcarboxamido}-5-trifluoromethyl-1,3,4-thiadiazole::CHEMBL87835
SMILES: O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(c2)C(=O)Nc2nnc(s2)C(F)(F)F)C(=O)N(Cc2cccc(c2)C(=O)Nc2nnc(s2)C(F)(F)F)[C@@H]1Cc1ccccc1
InChI Key: InChIKey=PYMPNOZOVIYDGG-ZRTHHSRSSA-N
Data: 2 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM50054170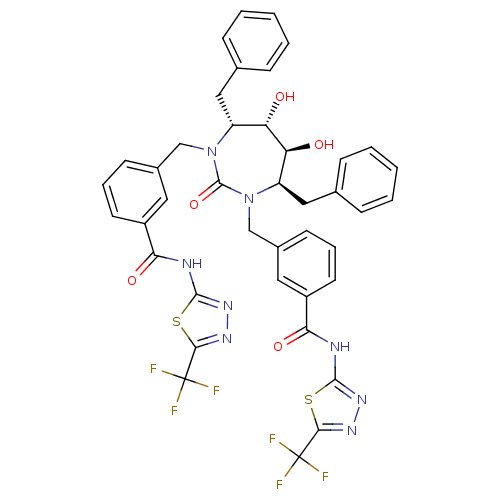 (2-{3-[4,7-dibenzyl-5,6-dihydroxy-2-oxo-3-[3-(5-tri...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lindsley F. Kimball Research Institute of The New York Blood Center Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 42: 249-59 (1999) Article DOI: 10.1021/jm980369n BindingDB Entry DOI: 10.7270/Q2JM28TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM50054170 (2-{3-[4,7-dibenzyl-5,6-dihydroxy-2-oxo-3-[3-(5-tri...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||