

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50054179 (4r,5r,6,7)-3,3-[[Tetrahydro-5,6-dihydroxy-2-oxo-4,7-bis(phenylmethyl)-1H-1,3-diazepine-1,3(2H)-diyl]bis-methylene)]bis[N-2-pyrazinylbenzamide]::2-{3-[4,7-dibenzyl-5,6-dihydroxy-2-oxo-3-[3-(2-pyrazinylcarbamoyl)benzyl]-(4R,5S,6S,7R)-1,3-diazepan-1-ylmethyl]phenylcarboxamido}pyrazine::CHEMBL262642
SMILES: O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(c2)C(=O)Nc2cnccn2)C(=O)N(Cc2cccc(c2)C(=O)Nc2cnccn2)[C@@H]1Cc1ccccc1
InChI Key: InChIKey=JIICMIOQFYIENG-JFDQHVTASA-N
Data: 4 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM50054179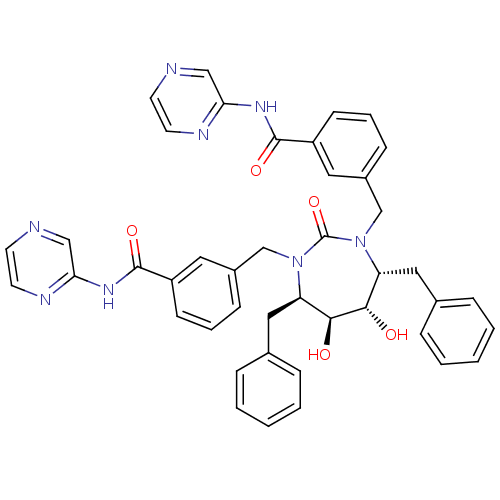 ((4r,5r,6,7)-3,3-[[Tetrahydro-5,6-dihydroxy-2-oxo-4...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM50054179 ((4r,5r,6,7)-3,3-[[Tetrahydro-5,6-dihydroxy-2-oxo-4...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lindsley F. Kimball Research Institute of The New York Blood Center Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 42: 249-59 (1999) Article DOI: 10.1021/jm980369n BindingDB Entry DOI: 10.7270/Q2JM28TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM50054179 ((4r,5r,6,7)-3,3-[[Tetrahydro-5,6-dihydroxy-2-oxo-4...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Affinity of the compound against HIV protease | J Med Chem 40: 4079-88 (1998) Article DOI: 10.1021/jm970288b BindingDB Entry DOI: 10.7270/Q2XS5TG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM50054179 ((4r,5r,6,7)-3,3-[[Tetrahydro-5,6-dihydroxy-2-oxo-4...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity for HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||