

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50055659 2N-(3,3-aminoiminopropyl)-4-[4-(4-formamido-1-methyl-1H-2-pyrrolylcarboxamido)-1-methyl-1H-2-pyrrolylcarboxamido]-1-methyl-1H-2-pyrrolecarboxamide::2N-[5-(3-amino-3-iminopropylcarbamoyl)-1-methyl-1H-3-pyrrolyl]-4-(4-formamido-1-methyl-1H-2-pyrrolylcarboxamido)-1-methyl-1H-2-pyrrolecarboxamide::2N-[5-(3-amino-3-iminopropylcarbamoyl)-1-methyl-1H-3-pyrrolyl]-4-(4-formamido-1-methyl-1H-2-pyrrolylcarboxamido)-1-methyl-1H-2-pyrrolecarboxamide(Distamycin)::2N-[5-(3-amino-3-iminopropylcarbamoyl)-1-methyl-1H-3-pyrrolyl]-4-(4-formamido-1-methyl-1H-2-pyrrolylcarboxamido)-1-methyl-1H-2-pyrrolecarboxamide(distamycin A)::4N-[5-(3-amino-3-iminopropylcarbamoyl)-1-methyl-1H-2-pyrrolyl]-2-(4-formamido-1-methyl-1H-2-pyrrolylcarboxamido)-1-methyl-1H-4-pyrrolecarboxamide::5N-[5-(3-amino-3-iminopropylcarbamoyl)-1-methyl-2,3-dihydro-1H-3-pyrrolyl]-3-(4-formamido-1-methyl-1H-2-pyrrolylcarboxamido)-1-methyl-2,3-dihydro-1H-5-pyrrolecarboxamide::CHEMBL101290::CHEMBL11252::DISTAMYCIN::DISTAMYCIN HYDROCHLORIDE::cid_6602691
SMILES: Cn1cc(NC=O)cc1C(=O)Nc1cc(C(=O)Nc2cc(C(=O)NCCC(N)=N)n(C)c2)n(C)c1
InChI Key: InChIKey=UPBAOYRENQEPJO-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM50055659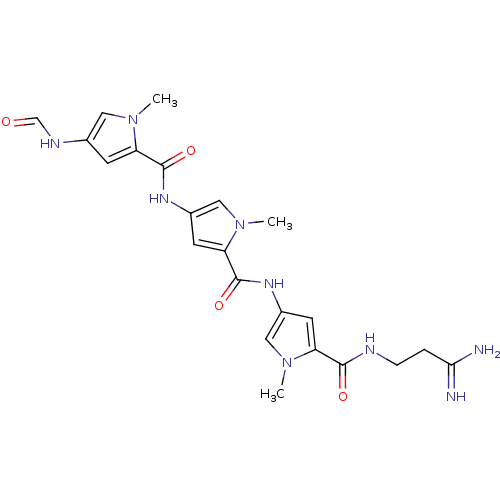 (2N-(3,3-aminoiminopropyl)-4-[4-(4-formamido-1-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PCMD Curated by PubChem BioAssay | Assay Description Project Title: A screen for modulators of human Rad51, a key DNA repair protein Application Number: MH084119 Assay Submitter: Dr. Alex Mazin Submitte... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2VD6WW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA repair protein RAD51 homolog 1 (Homo sapiens (Human)) | BDBM50055659 (2N-(3,3-aminoiminopropyl)-4-[4-(4-formamido-1-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | 6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PCMD Curated by PubChem BioAssay | Assay Description Project Title: A screen for modulators of human Rad51, a key DNA repair protein Application Number: MH084119 Assay Submitter: Dr. Alex Mazin Submitte... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2BV7F1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50055659 (2N-(3,3-aminoiminopropyl)-4-[4-(4-formamido-1-meth...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Inhibition of Ha-ras polymerase-chain reaction product | Bioorg Med Chem Lett 8: 3019-24 (1999) Article DOI: 10.1016/S0960-894X(98)00544-7 BindingDB Entry DOI: 10.7270/Q2057GFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase I (Topo I) (Homo sapiens (Human)) | BDBM50055659 (2N-(3,3-aminoiminopropyl)-4-[4-(4-formamido-1-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta Curated by ChEMBL | Assay Description Inhibit supercoil relaxation property of topoisomerase I. | J Med Chem 40: 216-25 (1997) Article DOI: 10.1021/jm9605804 BindingDB Entry DOI: 10.7270/Q2F47PS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50055659 (2N-(3,3-aminoiminopropyl)-4-[4-(4-formamido-1-meth...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50055659 (2N-(3,3-aminoiminopropyl)-4-[4-(4-formamido-1-meth...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Inhibition of Ha-ras polymerase-chain reaction product | Bioorg Med Chem Lett 8: 3019-24 (1999) Article DOI: 10.1016/S0960-894X(98)00544-7 BindingDB Entry DOI: 10.7270/Q2057GFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||