

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50057929 4-[2-(4-Chloro-phenyl)-4-trifluoromethyl-imidazol-1-yl]-benzenesulfonamide::CHEMBL45192
SMILES: NS(=O)(=O)c1ccc(cc1)-n1cc(nc1-c1ccc(Cl)cc1)C(F)(F)F
InChI Key: InChIKey=ZINNGAUEQDMHNV-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclooxygenase-1 (COX-1) (Homo sapiens (Human)) | BDBM50057929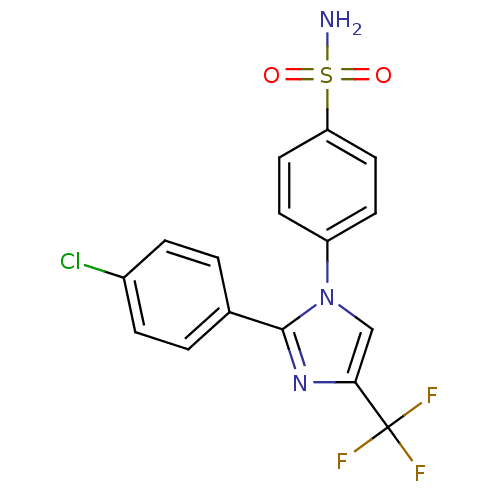 (4-[2-(4-Chloro-phenyl)-4-trifluoromethyl-imidazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit human prostaglandin G/H synthase 1 (COX-1) by 50% | J Med Chem 40: 1634-47 (1997) Article DOI: 10.1021/jm9700225 BindingDB Entry DOI: 10.7270/Q2F76BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase-2 (COX-2) (Mus musculus (Mouse)) | BDBM50057929 (4-[2-(4-Chloro-phenyl)-4-trifluoromethyl-imidazol-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hyderabad Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 2 (COX-2) | J Med Chem 45: 4847-57 (2002) BindingDB Entry DOI: 10.7270/Q2SX6FFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM50057929 (4-[2-(4-Chloro-phenyl)-4-trifluoromethyl-imidazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille 2 Curated by ChEMBL | Assay Description Inhibition of human Prostaglandin G/H synthase 2 | J Med Chem 44: 3223-30 (2001) BindingDB Entry DOI: 10.7270/Q2736S4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM50057929 (4-[2-(4-Chloro-phenyl)-4-trifluoromethyl-imidazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit human Prostaglandin G/H synthase 2 (COX-2) by 50% | J Med Chem 40: 1634-47 (1997) Article DOI: 10.1021/jm9700225 BindingDB Entry DOI: 10.7270/Q2F76BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||