 Found 8 hits for monomerid = 50059259
Found 8 hits for monomerid = 50059259 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2)
(Homo sapiens (Human)) | BDBM50059259
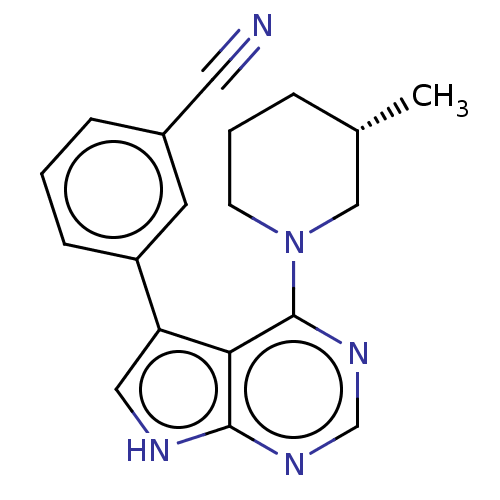
(CHEMBL3393341 | US9156845, 16)Show SMILES C[C@H]1CCCN(C1)c1ncnc2[nH]cc(-c3cccc(c3)C#N)c12 |r| Show InChI InChI=1S/C19H19N5/c1-13-4-3-7-24(11-13)19-17-16(10-21-18(17)22-12-23-19)15-6-2-5-14(8-15)9-20/h2,5-6,8,10,12-13H,3-4,7,11H2,1H3,(H,21,22,23)/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc.
US Patent
| Assay Description
LRRK2 kinase activity was measured using Lantha Screen technology from Invitrogen. GST-tagged truncated LRRK2 from Invitrogen (Cat # PV4874) was incu... |
US Patent US9156845 (2015)
BindingDB Entry DOI: 10.7270/Q2DB80MW |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2 G2019S
(Homo sapiens (Human)) | BDBM50059259

(CHEMBL3393341 | US9156845, 16)Show SMILES C[C@H]1CCCN(C1)c1ncnc2[nH]cc(-c3cccc(c3)C#N)c12 |r| Show InChI InChI=1S/C19H19N5/c1-13-4-3-7-24(11-13)19-17-16(10-21-18(17)22-12-23-19)15-6-2-5-14(8-15)9-20/h2,5-6,8,10,12-13H,3-4,7,11H2,1H3,(H,21,22,23)/t13-/m0/s1 | PDB
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc.
US Patent
| Assay Description
LRRK2 kinase activity was measured using Lantha Screen technology from Invitrogen. GST-tagged truncated LRRK2 from Invitrogen (Cat # PV4874) was incu... |
US Patent US9156845 (2015)
BindingDB Entry DOI: 10.7270/Q2DB80MW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50059259

(CHEMBL3393341 | US9156845, 16)Show SMILES C[C@H]1CCCN(C1)c1ncnc2[nH]cc(-c3cccc(c3)C#N)c12 |r| Show InChI InChI=1S/C19H19N5/c1-13-4-3-7-24(11-13)19-17-16(10-21-18(17)22-12-23-19)15-6-2-5-14(8-15)9-20/h2,5-6,8,10,12-13H,3-4,7,11H2,1H3,(H,21,22,23)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 (unknown origin) |
J Med Chem 58: 419-32 (2015)
Article DOI: 10.1021/jm5014055
BindingDB Entry DOI: 10.7270/Q2XS5X2W |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2)
(Homo sapiens (Human)) | BDBM50059259

(CHEMBL3393341 | US9156845, 16)Show SMILES C[C@H]1CCCN(C1)c1ncnc2[nH]cc(-c3cccc(c3)C#N)c12 |r| Show InChI InChI=1S/C19H19N5/c1-13-4-3-7-24(11-13)19-17-16(10-21-18(17)22-12-23-19)15-6-2-5-14(8-15)9-20/h2,5-6,8,10,12-13H,3-4,7,11H2,1H3,(H,21,22,23)/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged truncated human recombinant LRRK2 using fluorescein-labeled LRRKtide peptide substrate incubated for 2 hrs |
J Med Chem 58: 419-32 (2015)
Article DOI: 10.1021/jm5014055
BindingDB Entry DOI: 10.7270/Q2XS5X2W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50059259

(CHEMBL3393341 | US9156845, 16)Show SMILES C[C@H]1CCCN(C1)c1ncnc2[nH]cc(-c3cccc(c3)C#N)c12 |r| Show InChI InChI=1S/C19H19N5/c1-13-4-3-7-24(11-13)19-17-16(10-21-18(17)22-12-23-19)15-6-2-5-14(8-15)9-20/h2,5-6,8,10,12-13H,3-4,7,11H2,1H3,(H,21,22,23)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) |
J Med Chem 58: 419-32 (2015)
Article DOI: 10.1021/jm5014055
BindingDB Entry DOI: 10.7270/Q2XS5X2W |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2)
(Homo sapiens (Human)) | BDBM50059259

(CHEMBL3393341 | US9156845, 16)Show SMILES C[C@H]1CCCN(C1)c1ncnc2[nH]cc(-c3cccc(c3)C#N)c12 |r| Show InChI InChI=1S/C19H19N5/c1-13-4-3-7-24(11-13)19-17-16(10-21-18(17)22-12-23-19)15-6-2-5-14(8-15)9-20/h2,5-6,8,10,12-13H,3-4,7,11H2,1H3,(H,21,22,23)/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length LRRK2 (unknown origin) expressed in HEK293 cells assessed as reduction in S935 phosphorylation incubated for 90 mins by ELI... |
J Med Chem 58: 419-32 (2015)
Article DOI: 10.1021/jm5014055
BindingDB Entry DOI: 10.7270/Q2XS5X2W |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2)
(Homo sapiens (Human)) | BDBM50059259

(CHEMBL3393341 | US9156845, 16)Show SMILES C[C@H]1CCCN(C1)c1ncnc2[nH]cc(-c3cccc(c3)C#N)c12 |r| Show InChI InChI=1S/C19H19N5/c1-13-4-3-7-24(11-13)19-17-16(10-21-18(17)22-12-23-19)15-6-2-5-14(8-15)9-20/h2,5-6,8,10,12-13H,3-4,7,11H2,1H3,(H,21,22,23)/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged truncated human recombinant LRRK2 G2019S mutant using fluorescein-labeled LRRKtide peptide substrate incubated for 90 mins |
J Med Chem 58: 419-32 (2015)
Article DOI: 10.1021/jm5014055
BindingDB Entry DOI: 10.7270/Q2XS5X2W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50059259

(CHEMBL3393341 | US9156845, 16)Show SMILES C[C@H]1CCCN(C1)c1ncnc2[nH]cc(-c3cccc(c3)C#N)c12 |r| Show InChI InChI=1S/C19H19N5/c1-13-4-3-7-24(11-13)19-17-16(10-21-18(17)22-12-23-19)15-6-2-5-14(8-15)9-20/h2,5-6,8,10,12-13H,3-4,7,11H2,1H3,(H,21,22,23)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 575 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) |
J Med Chem 58: 419-32 (2015)
Article DOI: 10.1021/jm5014055
BindingDB Entry DOI: 10.7270/Q2XS5X2W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data