

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50059290 CHEMBL3393448::US9156845, 110
SMILES: Cc1noc(n1)C1CN(CCO1)c1ncnc2[nH]cc(-c3cccc(c3)C#N)c12
InChI Key: InChIKey=YSXZVUXTUVKHPF-UHFFFAOYSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2) (Homo sapiens (Human)) | BDBM50059290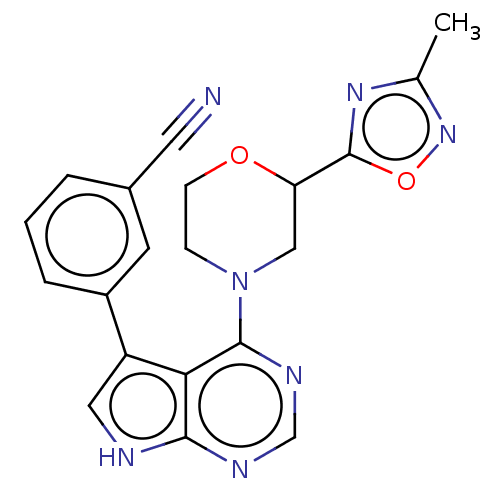 (CHEMBL3393448 | US9156845, 110) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. US Patent | Assay Description LRRK2 kinase activity was measured using Lantha Screen technology from Invitrogen. GST-tagged truncated LRRK2 from Invitrogen (Cat # PV4874) was incu... | US Patent US9156845 (2015) BindingDB Entry DOI: 10.7270/Q2DB80MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 G2019S (Homo sapiens (Human)) | BDBM50059290 (CHEMBL3393448 | US9156845, 110) | PDB GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 176 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. US Patent | Assay Description LRRK2 kinase activity was measured using Lantha Screen technology from Invitrogen. GST-tagged truncated LRRK2 from Invitrogen (Cat # PV4874) was incu... | US Patent US9156845 (2015) BindingDB Entry DOI: 10.7270/Q2DB80MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2) (Homo sapiens (Human)) | BDBM50059290 (CHEMBL3393448 | US9156845, 110) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged truncated human recombinant LRRK2 using fluorescein-labeled LRRKtide peptide substrate incubated for 2 hrs | J Med Chem 58: 419-32 (2015) Article DOI: 10.1021/jm5014055 BindingDB Entry DOI: 10.7270/Q2XS5X2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase MST2 (Homo sapiens (Human)) | BDBM50059290 (CHEMBL3393448 | US9156845, 110) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant MST2 using Ser/Thr peptide 7 substrate after 45 mins by Z-Lyte assay | J Med Chem 58: 419-32 (2015) Article DOI: 10.1021/jm5014055 BindingDB Entry DOI: 10.7270/Q2XS5X2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (LRRK2) (Homo sapiens (Human)) | BDBM50059290 (CHEMBL3393448 | US9156845, 110) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full length LRRK2 (unknown origin) expressed in HEK293 cells assessed as reduction in S935 phosphorylation incubated for 90 mins by ELI... | J Med Chem 58: 419-32 (2015) Article DOI: 10.1021/jm5014055 BindingDB Entry DOI: 10.7270/Q2XS5X2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase MST4 (Homo sapiens (Human)) | BDBM50059290 (CHEMBL3393448 | US9156845, 110) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant MST4 using Ser/Thr peptide 7 substrate after 60 mins by Z-Lyte assay | J Med Chem 58: 419-32 (2015) Article DOI: 10.1021/jm5014055 BindingDB Entry DOI: 10.7270/Q2XS5X2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||