 Found 5 hits for monomerid = 50059860
Found 5 hits for monomerid = 50059860 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Farnesyl diphosphate synthase
(Homo sapiens (Human)) | BDBM50059860
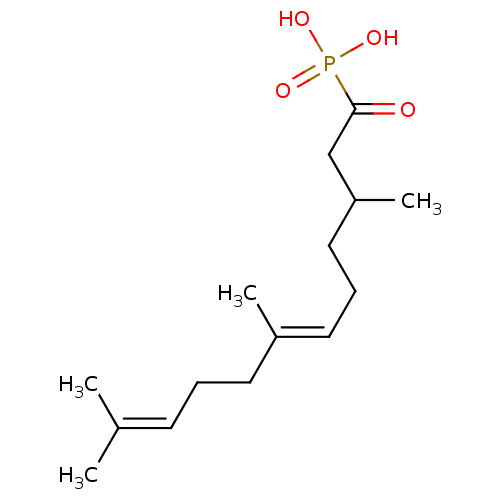
(((2E,6E)-1-Hydroxy-3,7,11-trimethyl-dodeca-2,6,10-...)Show SMILES [#6]-[#6](-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])-[#6]-[#6](=O)P([#8])([#8])=O Show InChI InChI=1S/C15H27O4P/c1-12(2)7-5-8-13(3)9-6-10-14(4)11-15(16)20(17,18)19/h7,9,14H,5-6,8,10-11H2,1-4H3,(H2,17,18,19)/b13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Assay using farnesyl-ras-CVLS as the protein acceptor substrate. |
Biochemistry 31: 3800-7 (1992)
Article DOI: 10.1021/bi00130a010
BindingDB Entry DOI: 10.7270/Q2MP51XH |
More data for this
Ligand-Target Pair | |
Protein Farnesyltransferase (PFT)
(Homo sapiens (Human)) | BDBM50059860

(((2E,6E)-1-Hydroxy-3,7,11-trimethyl-dodeca-2,6,10-...)Show SMILES [#6]-[#6](-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])-[#6]-[#6](=O)P([#8])([#8])=O Show InChI InChI=1S/C15H27O4P/c1-12(2)7-5-8-13(3)9-6-10-14(4)11-15(16)20(17,18)19/h7,9,14H,5-6,8,10-11H2,1-4H3,(H2,17,18,19)/b13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against farnesyl pyrophosphate |
J Med Chem 40: 2971-90 (1997)
Article DOI: 10.1021/jm970226l
BindingDB Entry DOI: 10.7270/Q26M37HF |
More data for this
Ligand-Target Pair | |
Farnesyl diphosphate synthase
(Homo sapiens (Human)) | BDBM50059860

(((2E,6E)-1-Hydroxy-3,7,11-trimethyl-dodeca-2,6,10-...)Show SMILES [#6]-[#6](-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])-[#6]-[#6](=O)P([#8])([#8])=O Show InChI InChI=1S/C15H27O4P/c1-12(2)7-5-8-13(3)9-6-10-14(4)11-15(16)20(17,18)19/h7,9,14H,5-6,8,10-11H2,1-4H3,(H2,17,18,19)/b13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
FPTase activity was assayed in the biosynthetically forward direction at 30C. |
Biochemistry 31: 3800-7 (1992)
Article DOI: 10.1021/bi00130a010
BindingDB Entry DOI: 10.7270/Q2MP51XH |
More data for this
Ligand-Target Pair | |
Protein Farnesyltransferase (PFT)
(Bos taurus (bovine)) | BDBM50059860

(((2E,6E)-1-Hydroxy-3,7,11-trimethyl-dodeca-2,6,10-...)Show SMILES [#6]-[#6](-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])-[#6]-[#6](=O)P([#8])([#8])=O Show InChI InChI=1S/C15H27O4P/c1-12(2)7-5-8-13(3)9-6-10-14(4)11-15(16)20(17,18)19/h7,9,14H,5-6,8,10-11H2,1-4H3,(H2,17,18,19)/b13-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of farnesyl protein transferase from bovine brain |
Bioorg Med Chem Lett 6: 1291-1296 (1996)
Article DOI: 10.1016/0960-894X(96)00238-7
BindingDB Entry DOI: 10.7270/Q2ZC82TQ |
More data for this
Ligand-Target Pair | |
Squalene synthetase
(Rattus norvegicus) | BDBM50059860

(((2E,6E)-1-Hydroxy-3,7,11-trimethyl-dodeca-2,6,10-...)Show SMILES [#6]-[#6](-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])-[#6]-[#6](=O)P([#8])([#8])=O Show InChI InChI=1S/C15H27O4P/c1-12(2)7-5-8-13(3)9-6-10-14(4)11-15(16)20(17,18)19/h7,9,14H,5-6,8,10-11H2,1-4H3,(H2,17,18,19)/b13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of squalene synthase from rat liver microsomes |
Bioorg Med Chem Lett 6: 1291-1296 (1996)
Article DOI: 10.1016/0960-894X(96)00238-7
BindingDB Entry DOI: 10.7270/Q2ZC82TQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data