

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50059985 1,7-Bis-(4-hydroxy-3-methoxy-phenyl)-heptane-3,5-dione::CHEMBL318743::tetrahydrocurcumin
SMILES: COc1cc(CCC(=O)CC(=O)CCc2ccc(O)c(OC)c2)ccc1O
InChI Key: InChIKey=LBTVHXHERHESKG-UHFFFAOYSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Integrase (Human immunodeficiency virus 1) | BDBM50059985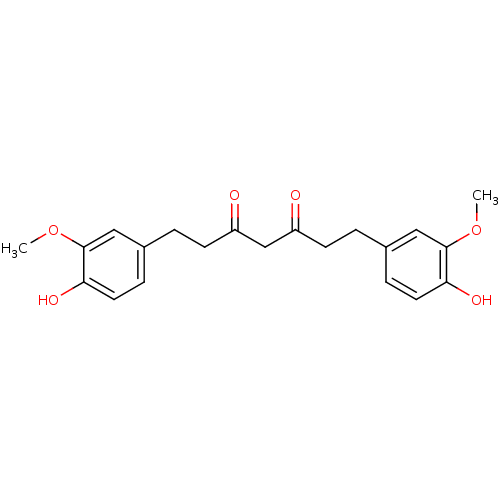 (1,7-Bis-(4-hydroxy-3-methoxy-phenyl)-heptane-3,5-d...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of 3'- processing activity of HIV-1 integrase | J Med Chem 40: 3057-63 (1997) Article DOI: 10.1021/jm970190x BindingDB Entry DOI: 10.7270/Q22Z166F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50059985 (1,7-Bis-(4-hydroxy-3-methoxy-phenyl)-heptane-3,5-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO expressed in Escherichia coli Bl21 (DE3) using arachidonic acid as substrate preincubated for 10 mins measured a... | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50059985 (1,7-Bis-(4-hydroxy-3-methoxy-phenyl)-heptane-3,5-d...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ramkhamhaeng University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem Lett 23: 2880-2 (2013) Article DOI: 10.1016/j.bmcl.2013.03.069 BindingDB Entry DOI: 10.7270/Q22Z16WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50059985 (1,7-Bis-(4-hydroxy-3-methoxy-phenyl)-heptane-3,5-d...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase | J Med Chem 40: 3057-63 (1997) Article DOI: 10.1021/jm970190x BindingDB Entry DOI: 10.7270/Q22Z166F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50059985 (1,7-Bis-(4-hydroxy-3-methoxy-phenyl)-heptane-3,5-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena Curated by ChEMBL | Assay Description Inhibition of STAT3 transcriptional activity in human HaCaT cells after 6 hrs by luciferase reporter gene assay | J Med Chem 57: 5638-48 (2014) Article DOI: 10.1021/jm500308c BindingDB Entry DOI: 10.7270/Q2HT2QWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||