

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50059989 (1E,4Z,6E)-5-Hydroxy-1,7-bis-(4-hydroxy-phenyl)-hepta-1,4,6-trien-3-one::(1E,6E)-1,7-Bis-(4-hydroxy-phenyl)-hepta-1,6-diene-3,5-dione::1,7-bis(4-hydroxyphenyl)-1,6-heptadiene-3,5-dione::1,7-bis(4-hydroxyphenyl)-3-hydroxy-1,3,6-heptatrien-5-one::5-Hydroxy-1,7-bis(4-hydroxyphenyl)hepta-1,4,6-trien-3-one::5-Hydroxy-1,7-bis-(4-hydroxy-phenyl)-hepta-1,4,6-trien-3-one::CHEMBL105350::CHEMBL131770::bis-demethoxycurcumin::cid_5324473::curcumin III
SMILES: Oc1ccc(C=CC(=O)CC(=O)C=Cc2ccc(O)cc2)cc1
InChI Key: InChIKey=PREBVFJICNPEKM-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glyoxalase 1 (GLO1) (Homo sapiens (Human)) | BDBM50059989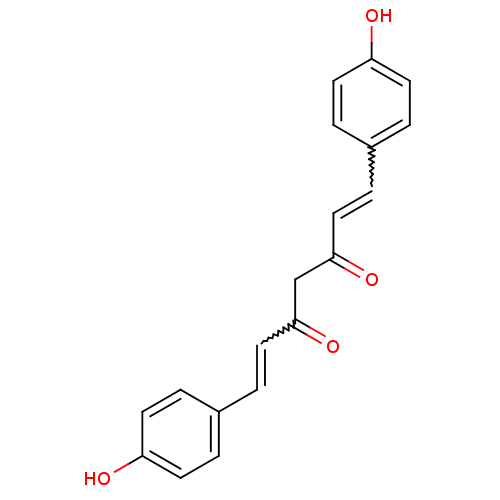 ((1E,4Z,6E)-5-Hydroxy-1,7-bis-(4-hydroxy-phenyl)-he...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged glyoxalase 1 expressed in Escherichia coli BL21 (DE3) preincubated for 20 mins by Dixon plot analysis | Bioorg Med Chem 19: 1189-96 (2011) Article DOI: 10.1016/j.bmc.2010.12.039 BindingDB Entry DOI: 10.7270/Q2222V2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glyoxalase 1 (GLO1) (Homo sapiens (Human)) | BDBM50059989 ((1E,4Z,6E)-5-Hydroxy-1,7-bis-(4-hydroxy-phenyl)-he...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-Sen University Curated by ChEMBL | Assay Description Inhibition of human glyoxalase 1 | Bioorg Med Chem Lett 21: 4243-7 (2011) Article DOI: 10.1016/j.bmcl.2011.05.095 BindingDB Entry DOI: 10.7270/Q23R0T76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM50059989 ((1E,4Z,6E)-5-Hydroxy-1,7-bis-(4-hydroxy-phenyl)-he...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of strand transfer activity of HIV-1 integrase | J Med Chem 40: 3057-63 (1997) Article DOI: 10.1021/jm970190x BindingDB Entry DOI: 10.7270/Q22Z166F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CAMK2A (Homo sapiens (Human)) | BDBM50059989 ((1E,4Z,6E)-5-Hydroxy-1,7-bis-(4-hydroxy-phenyl)-he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rajiv Gandhi Centre for Biotechnology Curated by ChEMBL | Assay Description Inhibition of alphaCaMK2 using GST-NR2A as substrate incubated for 1 min prior to substrate addition measured after 1 min | Bioorg Med Chem 20: 6040-7 (2012) Article DOI: 10.1016/j.bmc.2012.08.029 BindingDB Entry DOI: 10.7270/Q28916ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance protein CDR1 (Candida albicans) | BDBM50059989 ((1E,4Z,6E)-5-Hydroxy-1,7-bis-(4-hydroxy-phenyl)-he...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jawaharlal Nehru University Curated by ChEMBL | Assay Description Inhibition of GFP-tagged Candida albicans CDR1 expressed in Saccharomyces cerevisiae assessed as inhibition of R6G efflux | Antimicrob Agents Chemother 53: 3256-65 (2009) Article DOI: 10.1128/AAC.01497-08 BindingDB Entry DOI: 10.7270/Q2BP032R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM50059989 ((1E,4Z,6E)-5-Hydroxy-1,7-bis-(4-hydroxy-phenyl)-he...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of 3'- processing activity of HIV-1 integrase | J Med Chem 40: 3057-63 (1997) Article DOI: 10.1021/jm970190x BindingDB Entry DOI: 10.7270/Q22Z166F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM50059989 ((1E,4Z,6E)-5-Hydroxy-1,7-bis-(4-hydroxy-phenyl)-he...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Roma"La Sapienza" Curated by ChEMBL | Assay Description Compound concentration required to reduce HIV-1 Integrase 3'-processing activity by 50% | J Med Chem 41: 3948-60 (1998) Article DOI: 10.1021/jm9707232 BindingDB Entry DOI: 10.7270/Q29024G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM50059989 ((1E,4Z,6E)-5-Hydroxy-1,7-bis-(4-hydroxy-phenyl)-he...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Anti-oxidant activity in DPPH radicak scavenging assay; n=3-4 | Bioorg Med Chem Lett 15: 1793-7 (2005) Article DOI: 10.1016/j.bmcl.2005.02.039 BindingDB Entry DOI: 10.7270/Q20V8DJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50059989 ((1E,4Z,6E)-5-Hydroxy-1,7-bis-(4-hydroxy-phenyl)-he...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ramkhamhaeng University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Bioorg Med Chem Lett 23: 2880-2 (2013) Article DOI: 10.1016/j.bmcl.2013.03.069 BindingDB Entry DOI: 10.7270/Q22Z16WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M.SssI methyltransferase (Spiroplasma monobiae strain MQ-1) | BDBM50059989 ((1E,4Z,6E)-5-Hydroxy-1,7-bis-(4-hydroxy-phenyl)-he...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Inhibition of Spiroplasma sp. MQ-1 M.SssI | Bioorg Med Chem Lett 19: 706-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.041 BindingDB Entry DOI: 10.7270/Q2PV6M8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM50059989 ((1E,4Z,6E)-5-Hydroxy-1,7-bis-(4-hydroxy-phenyl)-he...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 Integrase (HIV-1-IN) | J Med Chem 45: 841-52 (2002) BindingDB Entry DOI: 10.7270/Q28W3FGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldose reductase (Homo sapiens (Human)) | BDBM50059989 ((1E,4Z,6E)-5-Hydroxy-1,7-bis-(4-hydroxy-phenyl)-he...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of AKR1B1 (unknown origin) | J Med Chem 60: 8441-8455 (2017) Article DOI: 10.1021/acs.jmedchem.7b00830 BindingDB Entry DOI: 10.7270/Q2NZ89S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family member 1B10 (AKR1B10) (Homo sapiens (Human)) | BDBM50059989 ((1E,4Z,6E)-5-Hydroxy-1,7-bis-(4-hydroxy-phenyl)-he...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of AKR1B10 (unknown origin) | J Med Chem 60: 8441-8455 (2017) Article DOI: 10.1021/acs.jmedchem.7b00830 BindingDB Entry DOI: 10.7270/Q2NZ89S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta lactamase (Pseudomonas aeruginosa) | BDBM50059989 ((1E,4Z,6E)-5-Hydroxy-1,7-bis-(4-hydroxy-phenyl)-he...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | 5.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Affiliation: The Scripps Research Institute, TSRI Assa... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2G15ZB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toll-like receptor 9 (Homo sapiens (Human)) | BDBM50059989 ((1E,4Z,6E)-5-Hydroxy-1,7-bis-(4-hydroxy-phenyl)-he...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | 9.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Affiliation: The Scripps Research Institute, TSRI Assa... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2KS6Q2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||