

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50060046 3-[(R)-2-[2-(7,7-Dimethyl-2-oxo-bicyclo[2.2.1]hept-1-yl)-ethanesulfonylamino]-3-(4-methanesulfonyl-piperazin-1-yl)-3-oxo-propyl]-benzamidine::CHEMBL319035
SMILES: CC1(C)C2CCC1(CCS(=O)(=O)N[C@H](Cc1cccc(c1)C(N)=N)C(=O)N1CCN(CC1)S(C)(=O)=O)C(=O)C2
InChI Key: InChIKey=JNPJLUMJGJWVKP-QLSBTUPBSA-N
Data: 10 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thrombin (Bos taurus (Bovine)) | BDBM50060046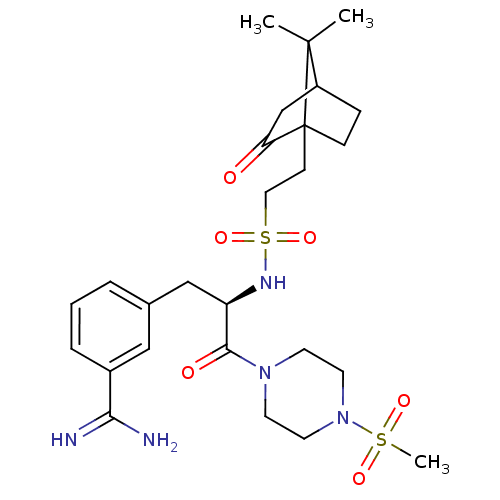 (3-[(R)-2-[2-(7,7-Dimethyl-2-oxo-bicyclo[2.2.1]hept...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Klinikum der Friedrich-Schiller-Universit£t Jena Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 40: 3091-9 (1997) Article DOI: 10.1021/jm960668h BindingDB Entry DOI: 10.7270/Q2Z60PRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin II (Bos taurus) | BDBM50060046 (3-[(R)-2-[2-(7,7-Dimethyl-2-oxo-bicyclo[2.2.1]hept...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Klinikum der Friedrich-Schiller-Universit£t Jena Curated by ChEMBL | Assay Description Inhibitory activity against trypsin | J Med Chem 40: 3091-9 (1997) Article DOI: 10.1021/jm960668h BindingDB Entry DOI: 10.7270/Q2Z60PRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Bos taurus) | BDBM50060046 (3-[(R)-2-[2-(7,7-Dimethyl-2-oxo-bicyclo[2.2.1]hept...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Klinikum der Friedrich-Schiller-Universit£t Jena Curated by ChEMBL | Assay Description Inhibitory activity against coagulation factor X. | J Med Chem 40: 3091-9 (1997) Article DOI: 10.1021/jm960668h BindingDB Entry DOI: 10.7270/Q2Z60PRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50060046 (3-[(R)-2-[2-(7,7-Dimethyl-2-oxo-bicyclo[2.2.1]hept...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Klinikum der Friedrich-Schiller-Universit£t Jena Curated by ChEMBL | Assay Description Inhibitory activity against Plasmin. | J Med Chem 40: 3091-9 (1997) Article DOI: 10.1021/jm960668h BindingDB Entry DOI: 10.7270/Q2Z60PRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50060046 (3-[(R)-2-[2-(7,7-Dimethyl-2-oxo-bicyclo[2.2.1]hept...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Klinikum der Friedrich-Schiller-Universit£t Jena Curated by ChEMBL | Assay Description Inhibitory activity against single-chain tissue-type plasminogen activator (sc-tissue plasminogen activator) | J Med Chem 40: 3091-9 (1997) Article DOI: 10.1021/jm960668h BindingDB Entry DOI: 10.7270/Q2Z60PRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50060046 (3-[(R)-2-[2-(7,7-Dimethyl-2-oxo-bicyclo[2.2.1]hept...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Klinikum der Friedrich-Schiller-Universit£t Jena Curated by ChEMBL | Assay Description Inhibitory activity against Kallikrein (PK) | J Med Chem 40: 3091-9 (1997) Article DOI: 10.1021/jm960668h BindingDB Entry DOI: 10.7270/Q2Z60PRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K-dependent protein C (Homo sapiens (Human)) | BDBM50060046 (3-[(R)-2-[2-(7,7-Dimethyl-2-oxo-bicyclo[2.2.1]hept...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Klinikum der Friedrich-Schiller-Universit£t Jena Curated by ChEMBL | Assay Description Inhibitory activity against Activated protein C | J Med Chem 40: 3091-9 (1997) Article DOI: 10.1021/jm960668h BindingDB Entry DOI: 10.7270/Q2Z60PRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50060046 (3-[(R)-2-[2-(7,7-Dimethyl-2-oxo-bicyclo[2.2.1]hept...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Klinikum der Friedrich-Schiller-Universit£t Jena Curated by ChEMBL | Assay Description Inhibitory activity against UK. | J Med Chem 40: 3091-9 (1997) Article DOI: 10.1021/jm960668h BindingDB Entry DOI: 10.7270/Q2Z60PRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Factor XIIa (Homo sapiens (Human)) | BDBM50060046 (3-[(R)-2-[2-(7,7-Dimethyl-2-oxo-bicyclo[2.2.1]hept...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Klinikum der Friedrich-Schiller-Universit£t Jena Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor XII | J Med Chem 40: 3091-9 (1997) Article DOI: 10.1021/jm960668h BindingDB Entry DOI: 10.7270/Q2Z60PRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein 1 (Homo sapiens (Human)) | BDBM50060046 (3-[(R)-2-[2-(7,7-Dimethyl-2-oxo-bicyclo[2.2.1]hept...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Klinikum der Friedrich-Schiller-Universit£t Jena Curated by ChEMBL | Assay Description Inhibitory activity against glandular kallikrein (GK) | J Med Chem 40: 3091-9 (1997) Article DOI: 10.1021/jm960668h BindingDB Entry DOI: 10.7270/Q2Z60PRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||