

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CN1c2ccccc2C(=N[C@@H](NC(=O)c2cc3ccccc3[nH]2)C1=O)c1ccccc1
InChI Key: InChIKey=NFHRQQKPEBFUJK-QHCPKHFHSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin receptor type A (RAT) | BDBM50060321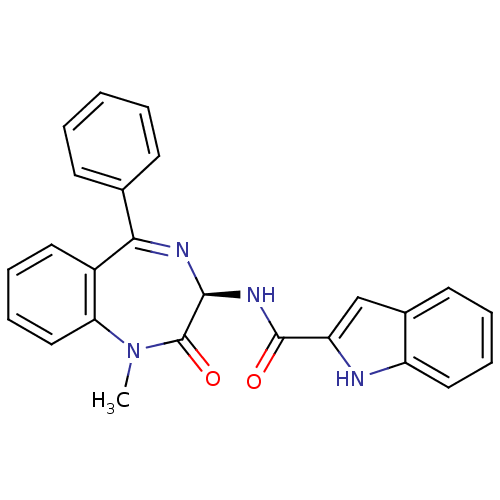 (1H-Indole-2-carboxylic acid ((R)-1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]-pCCK-8 specific binding to Cholecystokinin type A receptor of rat pancreas | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin (RAT) | BDBM50060321 (1H-Indole-2-carboxylic acid ((R)-1-methyl-2-oxo-5-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 83: 4918-22 (1986) Article DOI: 10.1073/pnas.83.13.4918 BindingDB Entry DOI: 10.7270/Q2445K05 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50060321 (1H-Indole-2-carboxylic acid ((R)-1-methyl-2-oxo-5-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 342 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibition of [3H]- pCCK-8 binding to Cholecystokinin type B receptor of rat cerebral cortex membranes | J Med Chem 40: 3402-7 (1997) Article DOI: 10.1021/jm9703247 BindingDB Entry DOI: 10.7270/Q2B56HV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA(A) receptor-associated protein (GUINEA PIG) | BDBM50060321 (1H-Indole-2-carboxylic acid ((R)-1-methyl-2-oxo-5-...) | PDB KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 83: 4918-22 (1986) Article DOI: 10.1073/pnas.83.13.4918 BindingDB Entry DOI: 10.7270/Q2445K05 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50060321 (1H-Indole-2-carboxylic acid ((R)-1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Research Laboratories Curated by ChEMBL | Assay Description Half-maximal inhibition of [125I]gastrin binding to guinea pig gastric glands | J Med Chem 31: 2235-46 (1989) BindingDB Entry DOI: 10.7270/Q2PG1S9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50060321 (1H-Indole-2-carboxylic acid ((R)-1-methyl-2-oxo-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Research Laboratories Curated by ChEMBL | Assay Description Half-maximal inhibition of [125I]-CCK-8(+) binding to cholecystokinin receptor from guinea pig brain tissue | J Med Chem 31: 2235-46 (1989) BindingDB Entry DOI: 10.7270/Q2PG1S9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50060321 (1H-Indole-2-carboxylic acid ((R)-1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [125I]-CCK-8 to Cholecystokinin type A receptor in rat pancreas | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50060321 (1H-Indole-2-carboxylic acid ((R)-1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig brain at a pH of 6.5 | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50060321 (1H-Indole-2-carboxylic acid ((R)-1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Half maximal inhibition of specific binding of [125I]-Bolton-Hunter CCK-8 to Cholecystokinin type A receptor in the rat pancreas | J Med Chem 43: 3505-17 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9XBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50060321 (1H-Indole-2-carboxylic acid ((R)-1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Half maximal inhibition of specific binding of [125I]Bolton-Hunter CCK-8 to Cholecystokinin type B receptor in the mouse cerebral cortex | J Med Chem 43: 3505-17 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9XBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50060321 (1H-Indole-2-carboxylic acid ((R)-1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Research Laboratories Curated by ChEMBL | Assay Description Half-maximal inhibition of [125I]CCK-8 binding to cholecystokinin receptor from rat pancreatic tissue | J Med Chem 31: 2235-46 (1989) BindingDB Entry DOI: 10.7270/Q2PG1S9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||