

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50060636 (S)-2-Amino-3-(5-bromo-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-propionic acid::(S)-2-amino-3-(5-bromo-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)propanoic acid::2-AMINO-3-(5-BROMO-2,4-DIOXO-3,4-DIHYDRO-2H-PYRIMIDIN-1-YL)-PROPIONIC ACID::CHEMBL333964
SMILES: N[C@@H](Cn1cc(Br)c(=O)[nH]c1=O)C(O)=O
InChI Key: InChIKey=AEKIJKSVXKWGRJ-BYPYZUCNSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grik5 (Homo sapiens (Human)) | BDBM50060636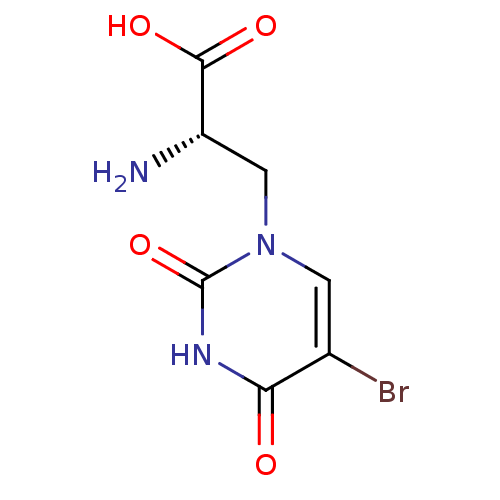 ((S)-2-Amino-3-(5-bromo-2,4-dioxo-3,4-dihydro-2H-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Displacement of [3H]kainate from human Ionotropic glutamate receptor ionotropic kainate 1 expressed in HEK293 cells | J Med Chem 40: 3645-50 (1997) Article DOI: 10.1021/jm9702387 BindingDB Entry DOI: 10.7270/Q28G8MCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GRIA1 (Homo sapiens (Human)) | BDBM50060636 ((S)-2-Amino-3-(5-bromo-2,4-dioxo-3,4-dihydro-2H-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from human Ionotropic glutamate receptor AMPA 1 expressed in HEK293 cells | J Med Chem 40: 3645-50 (1997) Article DOI: 10.1021/jm9702387 BindingDB Entry DOI: 10.7270/Q28G8MCB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| GRIA2 (Homo sapiens (Human)) | BDBM50060636 ((S)-2-Amino-3-(5-bromo-2,4-dioxo-3,4-dihydro-2H-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from human Ionotropic glutamate receptor AMPA 2 expressed in HEK293 cells | J Med Chem 40: 3645-50 (1997) Article DOI: 10.1021/jm9702387 BindingDB Entry DOI: 10.7270/Q28G8MCB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic AMPA (Homo sapiens (Human)) | BDBM50060636 ((S)-2-Amino-3-(5-bromo-2,4-dioxo-3,4-dihydro-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 457 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from human Ionotropic glutamate receptor AMPA 4 expressed in HEK293 cells | J Med Chem 40: 3645-50 (1997) Article DOI: 10.1021/jm9702387 BindingDB Entry DOI: 10.7270/Q28G8MCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 1 (Rattus norvegicus (Rat)) | BDBM50060636 ((S)-2-Amino-3-(5-bromo-2,4-dioxo-3,4-dihydro-2H-py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a |
European Research Centre for Drug Discovery and Development (NatSynDrugs) Curated by ChEMBL | Assay Description Agonist activity at cyclothiazide-desensitized rat recombinant flop iGluR1 expressed in Xenopus laevis oocytes by two electrode voltage-clamp electro... | J Med Chem 51: 6614-8 (2008) Article DOI: 10.1021/jm800865a BindingDB Entry DOI: 10.7270/Q261117R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||