

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50060707 (S)-1-(3-Phenyl-3-pyridin-3-yl-propionyl)-pyrrolidine-2-carboxylic acid (4-amino-cyclohexylmethyl)-amide; TFA::CHEMBL333238
SMILES: NC1CCC(CNC(=O)[C@@H]2CCCN2C(=O)CC(c2ccccc2)c2cccnc2)CC1
InChI Key: InChIKey=RSQVIWGRLAYAKO-NCXVZUCISA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50060707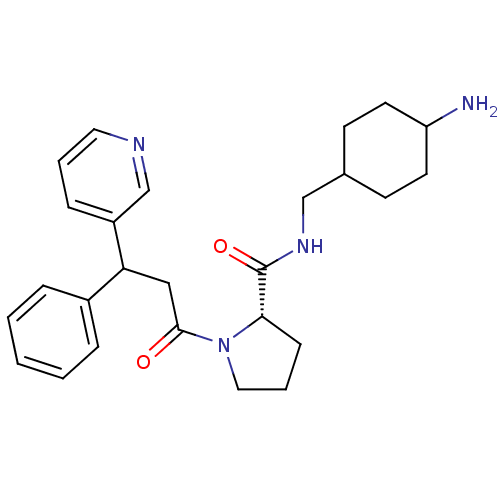 ((S)-1-(3-Phenyl-3-pyridin-3-yl-propionyl)-pyrrolid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | 2.10E+3 | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Thrombin | J Med Chem 40: 3687-93 (1997) Article DOI: 10.1021/jm970397q BindingDB Entry DOI: 10.7270/Q20Z73ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin-1 (Homo sapiens (Human)) | BDBM50060707 ((S)-1-(3-Phenyl-3-pyridin-3-yl-propionyl)-pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of human trypsin. | J Med Chem 40: 3687-93 (1997) Article DOI: 10.1021/jm970397q BindingDB Entry DOI: 10.7270/Q20Z73ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50060707 ((S)-1-(3-Phenyl-3-pyridin-3-yl-propionyl)-pyrrolid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.76E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against serine protease plasma kallikrein | J Med Chem 40: 3687-93 (1997) Article DOI: 10.1021/jm970397q BindingDB Entry DOI: 10.7270/Q20Z73ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50060707 ((S)-1-(3-Phenyl-3-pyridin-3-yl-propionyl)-pyrrolid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.46E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against serine protease TPA | J Med Chem 40: 3687-93 (1997) Article DOI: 10.1021/jm970397q BindingDB Entry DOI: 10.7270/Q20Z73ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50060707 ((S)-1-(3-Phenyl-3-pyridin-3-yl-propionyl)-pyrrolid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against serine protease plasmin | J Med Chem 40: 3687-93 (1997) Article DOI: 10.1021/jm970397q BindingDB Entry DOI: 10.7270/Q20Z73ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50060707 ((S)-1-(3-Phenyl-3-pyridin-3-yl-propionyl)-pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against serine protease Coagulation factor X | J Med Chem 40: 3687-93 (1997) Article DOI: 10.1021/jm970397q BindingDB Entry DOI: 10.7270/Q20Z73ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||