 Found 5 hits for monomerid = 50061990
Found 5 hits for monomerid = 50061990 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholecystokinin receptor
(MOUSE) | BDBM50061990
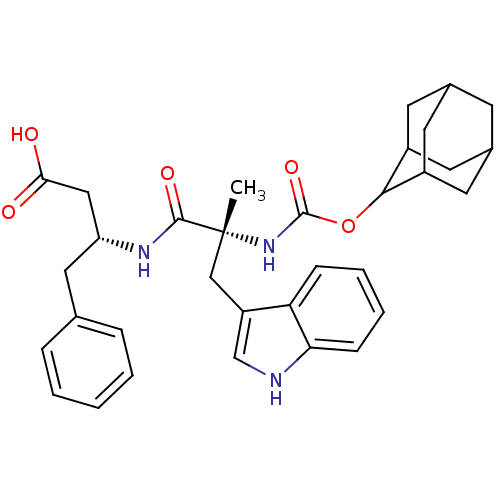
((R)-3-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N[C@@H](CC(O)=O)Cc1ccccc1 |wU:1.13,29.33,wD:1.0,TLB:15:16:18:21.22.20,THB:23:24:18:21.22.20,23:21:18:16.24.25,20:21:16:19.18.25,20:19:16:21.23.22,(10.8,-2.25,;10.81,-3.63,;10.9,-5.18,;12.26,-5.88,;11.02,-6.79,;11.49,-8.25,;13.03,-8.24,;14.06,-9.39,;15.56,-9.07,;16.04,-7.61,;15.01,-6.46,;13.52,-6.79,;9.43,-3.12,;8.15,-3.96,;8.17,-5.43,;6.76,-3.28,;5.47,-4.12,;5.46,-5.67,;4.44,-6.95,;3.04,-6.37,;1.53,-6.79,;2.74,-5.52,;4.05,-6.01,;2.72,-4.03,;4.08,-3.55,;3.02,-4.78,;12.1,-2.95,;12.07,-1.57,;13.48,-3.63,;14.76,-2.78,;14.67,-1.25,;15.43,.09,;14.65,1.43,;16.98,.11,;16.14,-3.47,;17.43,-2.62,;17.34,-1.25,;18.6,-.23,;20,-.92,;20.1,-2.46,;18.81,-3.31,)| Show InChI InChI=1S/C33H39N3O5/c1-33(18-25-19-34-28-10-6-5-9-27(25)28,31(39)35-26(17-29(37)38)16-20-7-3-2-4-8-20)36-32(40)41-30-23-12-21-11-22(14-23)15-24(30)13-21/h2-10,19,21-24,26,30,34H,11-18H2,1H3,(H,35,39)(H,36,40)(H,37,38)/t21?,22?,23?,24?,26-,30?,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Cholecystokinin type B receptor binding assay performed on homogenized cerebral cortex from male mouse |
J Med Chem 35: 1572-7 (1992)
BindingDB Entry DOI: 10.7270/Q26H4KM8 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor
(MOUSE) | BDBM50061990

((R)-3-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N[C@@H](CC(O)=O)Cc1ccccc1 |wU:1.13,29.33,wD:1.0,TLB:15:16:18:21.22.20,THB:23:24:18:21.22.20,23:21:18:16.24.25,20:21:16:19.18.25,20:19:16:21.23.22,(10.8,-2.25,;10.81,-3.63,;10.9,-5.18,;12.26,-5.88,;11.02,-6.79,;11.49,-8.25,;13.03,-8.24,;14.06,-9.39,;15.56,-9.07,;16.04,-7.61,;15.01,-6.46,;13.52,-6.79,;9.43,-3.12,;8.15,-3.96,;8.17,-5.43,;6.76,-3.28,;5.47,-4.12,;5.46,-5.67,;4.44,-6.95,;3.04,-6.37,;1.53,-6.79,;2.74,-5.52,;4.05,-6.01,;2.72,-4.03,;4.08,-3.55,;3.02,-4.78,;12.1,-2.95,;12.07,-1.57,;13.48,-3.63,;14.76,-2.78,;14.67,-1.25,;15.43,.09,;14.65,1.43,;16.98,.11,;16.14,-3.47,;17.43,-2.62,;17.34,-1.25,;18.6,-.23,;20,-.92,;20.1,-2.46,;18.81,-3.31,)| Show InChI InChI=1S/C33H39N3O5/c1-33(18-25-19-34-28-10-6-5-9-27(25)28,31(39)35-26(17-29(37)38)16-20-7-3-2-4-8-20)36-32(40)41-30-23-12-21-11-22(14-23)15-24(30)13-21/h2-10,19,21-24,26,30,34H,11-18H2,1H3,(H,35,39)(H,36,40)(H,37,38)/t21?,22?,23?,24?,26-,30?,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-CCK-8 to Cholecystokinin type B receptor in the mouse cerebral cortex. |
Bioorg Med Chem Lett 3: 881-884 (1993)
Article DOI: 10.1016/S0960-894X(00)80685-X
BindingDB Entry DOI: 10.7270/Q2WM1DC5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor
(RAT) | BDBM50061990

((R)-3-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N[C@@H](CC(O)=O)Cc1ccccc1 |wU:1.13,29.33,wD:1.0,TLB:15:16:18:21.22.20,THB:23:24:18:21.22.20,23:21:18:16.24.25,20:21:16:19.18.25,20:19:16:21.23.22,(10.8,-2.25,;10.81,-3.63,;10.9,-5.18,;12.26,-5.88,;11.02,-6.79,;11.49,-8.25,;13.03,-8.24,;14.06,-9.39,;15.56,-9.07,;16.04,-7.61,;15.01,-6.46,;13.52,-6.79,;9.43,-3.12,;8.15,-3.96,;8.17,-5.43,;6.76,-3.28,;5.47,-4.12,;5.46,-5.67,;4.44,-6.95,;3.04,-6.37,;1.53,-6.79,;2.74,-5.52,;4.05,-6.01,;2.72,-4.03,;4.08,-3.55,;3.02,-4.78,;12.1,-2.95,;12.07,-1.57,;13.48,-3.63,;14.76,-2.78,;14.67,-1.25,;15.43,.09,;14.65,1.43,;16.98,.11,;16.14,-3.47,;17.43,-2.62,;17.34,-1.25,;18.6,-.23,;20,-.92,;20.1,-2.46,;18.81,-3.31,)| Show InChI InChI=1S/C33H39N3O5/c1-33(18-25-19-34-28-10-6-5-9-27(25)28,31(39)35-26(17-29(37)38)16-20-7-3-2-4-8-20)36-32(40)41-30-23-12-21-11-22(14-23)15-24(30)13-21/h2-10,19,21-24,26,30,34H,11-18H2,1H3,(H,35,39)(H,36,40)(H,37,38)/t21?,22?,23?,24?,26-,30?,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against radioligand [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type A receptor in the rat pancreas |
J Med Chem 41: 38-45 (1998)
Article DOI: 10.1021/jm970065l
BindingDB Entry DOI: 10.7270/Q2GT5NV6 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor
(RAT) | BDBM50061990

((R)-3-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N[C@@H](CC(O)=O)Cc1ccccc1 |wU:1.13,29.33,wD:1.0,TLB:15:16:18:21.22.20,THB:23:24:18:21.22.20,23:21:18:16.24.25,20:21:16:19.18.25,20:19:16:21.23.22,(10.8,-2.25,;10.81,-3.63,;10.9,-5.18,;12.26,-5.88,;11.02,-6.79,;11.49,-8.25,;13.03,-8.24,;14.06,-9.39,;15.56,-9.07,;16.04,-7.61,;15.01,-6.46,;13.52,-6.79,;9.43,-3.12,;8.15,-3.96,;8.17,-5.43,;6.76,-3.28,;5.47,-4.12,;5.46,-5.67,;4.44,-6.95,;3.04,-6.37,;1.53,-6.79,;2.74,-5.52,;4.05,-6.01,;2.72,-4.03,;4.08,-3.55,;3.02,-4.78,;12.1,-2.95,;12.07,-1.57,;13.48,-3.63,;14.76,-2.78,;14.67,-1.25,;15.43,.09,;14.65,1.43,;16.98,.11,;16.14,-3.47,;17.43,-2.62,;17.34,-1.25,;18.6,-.23,;20,-.92,;20.1,-2.46,;18.81,-3.31,)| Show InChI InChI=1S/C33H39N3O5/c1-33(18-25-19-34-28-10-6-5-9-27(25)28,31(39)35-26(17-29(37)38)16-20-7-3-2-4-8-20)36-32(40)41-30-23-12-21-11-22(14-23)15-24(30)13-21/h2-10,19,21-24,26,30,34H,11-18H2,1H3,(H,35,39)(H,36,40)(H,37,38)/t21?,22?,23?,24?,26-,30?,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-CCK-8 binding to Cholecystokinin type A receptor of rat pancreas |
Bioorg Med Chem Lett 3: 881-884 (1993)
Article DOI: 10.1016/S0960-894X(00)80685-X
BindingDB Entry DOI: 10.7270/Q2WM1DC5 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor
(MOUSE) | BDBM50061990

((R)-3-[(R)-2-(Adamantan-2-yloxycarbonylamino)-3-(1...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N[C@@H](CC(O)=O)Cc1ccccc1 |wU:1.13,29.33,wD:1.0,TLB:15:16:18:21.22.20,THB:23:24:18:21.22.20,23:21:18:16.24.25,20:21:16:19.18.25,20:19:16:21.23.22,(10.8,-2.25,;10.81,-3.63,;10.9,-5.18,;12.26,-5.88,;11.02,-6.79,;11.49,-8.25,;13.03,-8.24,;14.06,-9.39,;15.56,-9.07,;16.04,-7.61,;15.01,-6.46,;13.52,-6.79,;9.43,-3.12,;8.15,-3.96,;8.17,-5.43,;6.76,-3.28,;5.47,-4.12,;5.46,-5.67,;4.44,-6.95,;3.04,-6.37,;1.53,-6.79,;2.74,-5.52,;4.05,-6.01,;2.72,-4.03,;4.08,-3.55,;3.02,-4.78,;12.1,-2.95,;12.07,-1.57,;13.48,-3.63,;14.76,-2.78,;14.67,-1.25,;15.43,.09,;14.65,1.43,;16.98,.11,;16.14,-3.47,;17.43,-2.62,;17.34,-1.25,;18.6,-.23,;20,-.92,;20.1,-2.46,;18.81,-3.31,)| Show InChI InChI=1S/C33H39N3O5/c1-33(18-25-19-34-28-10-6-5-9-27(25)28,31(39)35-26(17-29(37)38)16-20-7-3-2-4-8-20)36-32(40)41-30-23-12-21-11-22(14-23)15-24(30)13-21/h2-10,19,21-24,26,30,34H,11-18H2,1H3,(H,35,39)(H,36,40)(H,37,38)/t21?,22?,23?,24?,26-,30?,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against radioligand [125I]Bolton-Hunter labeled CCK-8 to cholecystokinin type B receptor in the mouse cerebral cortex |
J Med Chem 41: 38-45 (1998)
Article DOI: 10.1021/jm970065l
BindingDB Entry DOI: 10.7270/Q2GT5NV6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data




