

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50063910 (R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propyl)-N*4*-hydroxy-2-(3-phenyl-propyl)-succinamide::(R)-N1-((S)-3,3-dimethyl-1-(methylamino)-1-oxobutan-2-yl)-N4-hydroxy-2-(3-phenylpropyl)succinamide::CHEMBL20154
SMILES: CNC(=O)[C@@H](NC(=O)[C@H](CCCc1ccccc1)CC(=O)NO)C(C)(C)C
InChI Key: InChIKey=LPZWIAVKYXCHGC-NVXWUHKLSA-N
Data: 9 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Matrix metalloproteinase-14 (MMP14) (Homo sapiens (Human)) | BDBM50063910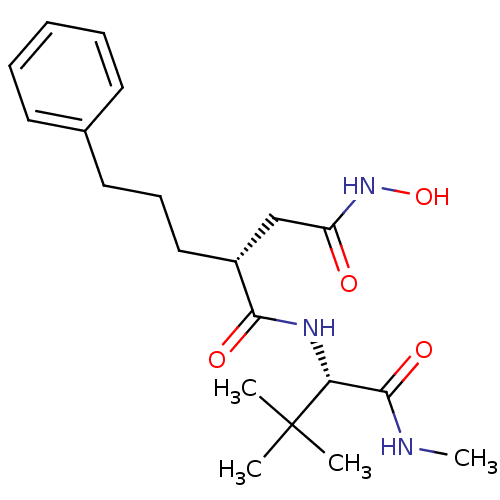 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd. Curated by ChEMBL | Assay Description Activity against deletion mutant of MT1-MMP lacking the transmembrane domain (deltaMT1) | J Med Chem 41: 1209-17 (1998) Article DOI: 10.1021/jm970404a BindingDB Entry DOI: 10.7270/Q2SQ8ZHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50063910 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd. Curated by ChEMBL | Assay Description Activity against Matrix metalloproteinase-9 (MMP-9). | J Med Chem 41: 1209-17 (1998) Article DOI: 10.1021/jm970404a BindingDB Entry DOI: 10.7270/Q2SQ8ZHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50063910 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd. Curated by ChEMBL | Assay Description Activity against Matrix metalloproteinase-1 (MMP-1). | J Med Chem 41: 1209-17 (1998) Article DOI: 10.1021/jm970404a BindingDB Entry DOI: 10.7270/Q2SQ8ZHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50063910 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-2 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50063910 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.339 | n/a | n/a | n/a | n/a | n/a | n/a |
Pomona College Curated by ChEMBL | Assay Description Inhibition of MMP2 | Bioorg Med Chem 15: 2223-68 (2007) Article DOI: 10.1016/j.bmc.2007.01.011 BindingDB Entry DOI: 10.7270/Q2571DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50063910 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-2 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50063910 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit the catalytic domain Matrix metalloprotease-3 using Nagase fluorogenic as a substrate. | Bioorg Med Chem Lett 11: 571-4 (2001) BindingDB Entry DOI: 10.7270/Q22B8X9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50063910 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Pomona College Curated by ChEMBL | Assay Description Inhibition of MMP3 | Bioorg Med Chem 15: 2223-68 (2007) Article DOI: 10.1016/j.bmc.2007.01.011 BindingDB Entry DOI: 10.7270/Q2571DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50063910 ((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Ability to inhibit the matrix metalloprotease-3 by method of Knight et al using the fluorogenic peptide substrate. | Bioorg Med Chem Lett 11: 567-70 (2001) BindingDB Entry DOI: 10.7270/Q2610ZK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||