

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50065120 CHEMBL302709::N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][1,4]diazepine-10-carbonyl)-phenyl]-2-chloro-benzamide
SMILES: Clc1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1Cc2cccn2Cc2ccccc12
InChI Key: InChIKey=UMCJOYNETNUIDU-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasopressin V2 Receptor (Rattus norvegicus (Rat)) | BDBM50065120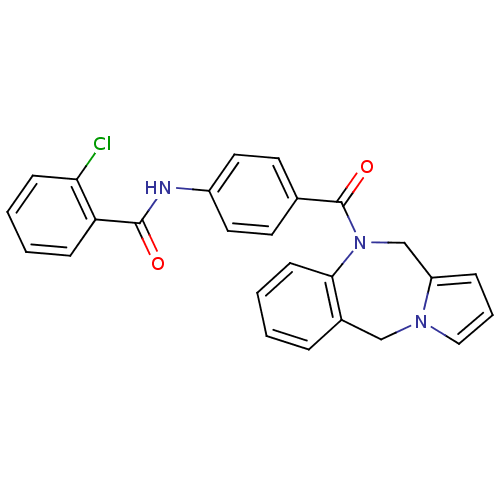 (CHEMBL302709 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from vasopressin V2 receptor of rat kidney medulla. | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 Receptor (Rattus norvegicus (Rat)) | BDBM50065120 (CHEMBL302709 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat kidney medullary vasopressin V2 receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| AVPR1A (RAT) | BDBM50065120 (CHEMBL302709 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat hepatic vasopressin V1a receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| AVPR1A (RAT) | BDBM50065120 (CHEMBL302709 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of (Phe-3,4,5-H) Vasopressin from V1a recptor from rat liver membranes. | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||