

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50065468 2-(4-Chloro-phenoxymethyl)-4-(3-piperidin-1-yl-propoxy)-1-(3-piperidin-3-yl-propyl)-1H-benzoimidazole::CHEMBL61532
SMILES: Clc1ccc(OCc2nc3c(OCCCN4CCCCC4)cccc3n2CCCC2CCCNC2)cc1
InChI Key: InChIKey=SHFZELZOQAXTFF-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065468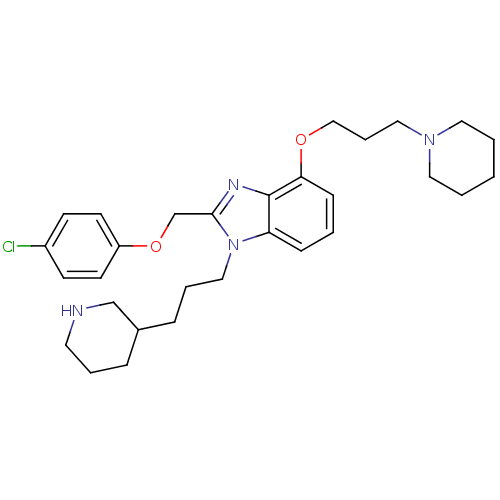 (2-(4-Chloro-phenoxymethyl)-4-(3-piperidin-1-yl-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human neuropeptide Y receptor type 1, determined by measuring its ability to displace [125]peptide YY | J Med Chem 41: 2709-19 (1998) Article DOI: 10.1021/jm9706630 BindingDB Entry DOI: 10.7270/Q2TD9WGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065468 (2-(4-Chloro-phenoxymethyl)-4-(3-piperidin-1-yl-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells | Bioorg Med Chem Lett 9: 647-52 (1999) BindingDB Entry DOI: 10.7270/Q2QN65ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50065468 (2-(4-Chloro-phenoxymethyl)-4-(3-piperidin-1-yl-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Binding affinity of the compound at cloned Neuropeptide Y receptor type 1 expressed in AV-12 cells is evaluated. | Bioorg Med Chem Lett 8: 473-6 (1999) BindingDB Entry DOI: 10.7270/Q2GH9H33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (RAT) | BDBM50065468 (2-(4-Chloro-phenoxymethyl)-4-(3-piperidin-1-yl-pro...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description The compound was tested for the binding affinity against Neuropeptide Y receptor type 1 in rat | J Med Chem 42: 181-201 (1999) Article DOI: 10.1021/jm980521l BindingDB Entry DOI: 10.7270/Q2T43S8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||