

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: OC(=O)CCCC(=O)Nc1nc2ccc(Cl)cc2c2nc(nn12)-c1ccco1
InChI Key: InChIKey=JTVWWLKUEHXOQG-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50065771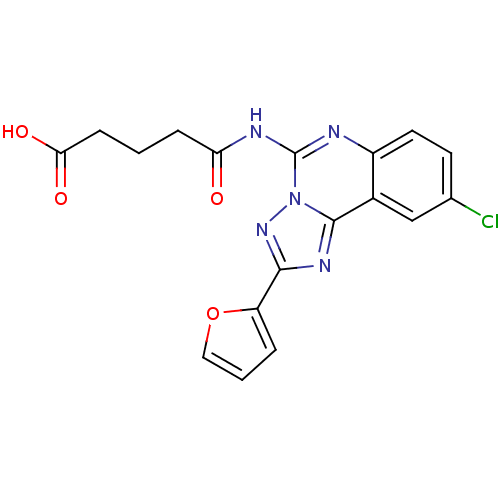 (4-(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]qui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity was determined in radioligand binding assay at rat striatal Adenosine A2A receptor vs [3H]-CGS- 21680 | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50065771 (4-(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]qui...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity was determined in radioligand binding assay at rat brain adenosine A1 receptor vs [3H]-R-PIA | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50065771 (4-(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]qui...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 81.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity at cloned human adenosine A3 receptor expressed in HEK-293 cells was determined using [125I]-AB-MECA as radioligand | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50065771 (4-(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]qui...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Inhibition of 50 uM 5`-(N-ethylcarboxamido)adenosine induced cAMP production in CHO cell line expressing human Adenosine A2B receptor at 50 uM | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||