

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50066066 (S)-1-[4-(Benzothiazol-2-yl-methyl-amino)-piperidin-1-yl]-3-(3,4-difluoro-phenoxy)-propan-2-ol::1-[4-(Benzothiazol-2-yl-methyl-amino)-piperidin-1-yl]-3-(3,4-difluoro-phenoxy)-propan-2-ol(Lubeluzole)::CHEMBL281724::LUBELUZOLE
SMILES: CN(C1CCN(C[C@H](O)COc2ccc(F)c(F)c2)CC1)c1nc2ccccc2s1
InChI Key: InChIKey=OZFSWVOEXHGDES-INIZCTEOSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium channel protein type I I alpha subunit (Rattus norvegicus) | BDBM50066066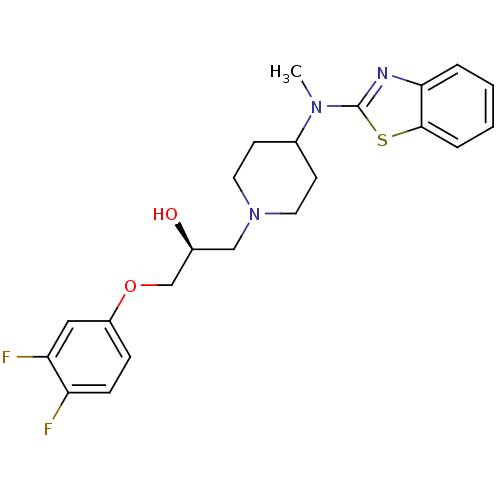 ((S)-1-[4-(Benzothiazol-2-yl-methyl-amino)-piperidi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma KG Curated by ChEMBL | Assay Description Displacement of [3H]BTX from sodium channel of rat cerebral cortex synaptosomes | J Med Chem 45: 3755-64 (2002) Article DOI: 10.1021/jm020875j BindingDB Entry DOI: 10.7270/Q2ZS308Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type I I alpha subunit (Rattus norvegicus) | BDBM50066066 ((S)-1-[4-(Benzothiazol-2-yl-methyl-amino)-piperidi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma KG Curated by ChEMBL | Assay Description Inhibitory effect against veratridine-induced glutamate release from rat brain slices | J Med Chem 45: 3755-64 (2002) Article DOI: 10.1021/jm020875j BindingDB Entry DOI: 10.7270/Q2ZS308Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calmodulin (Bos taurus) | BDBM50066066 ((S)-1-[4-(Benzothiazol-2-yl-methyl-amino)-piperidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari 'Aldo Moro' Curated by ChEMBL | Assay Description Binding affinity to bovine brain CaM by FTPFACE analysis | Eur J Med Chem 116: 36-45 (2016) BindingDB Entry DOI: 10.7270/Q2Q2424Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type II alpha subunit (Rattus norvegicus) | BDBM50066066 ((S)-1-[4-(Benzothiazol-2-yl-methyl-amino)-piperidi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc. Curated by ChEMBL | Assay Description In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) | J Med Chem 41: 3048-61 (1998) Article DOI: 10.1021/jm980124a BindingDB Entry DOI: 10.7270/Q2R49RF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||