

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50066470 ((S)-1-{N'-[4-(2-tert-Butyl-2H-tetrazol-5-yl)-benzyl]-N'-[(2S,3S)-2-hydroxy-3-((S)-2-methoxycarbonylamino-3,3-dimethyl-butyrylamino)-4-phenyl-butyl]-hydrazinocarbonyl}-2,2-dimethyl-propyl)-carbamic acid methyl ester::CHEMBL325271
SMILES: COC(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CN(Cc1ccc(cc1)-c1nnn(n1)C(C)(C)C)NC(=O)[C@@H](NC(=O)OC)C(C)(C)C)C(C)(C)C
InChI Key: InChIKey=RXWRDDXNHGYOJF-VZNYXHRGSA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50066470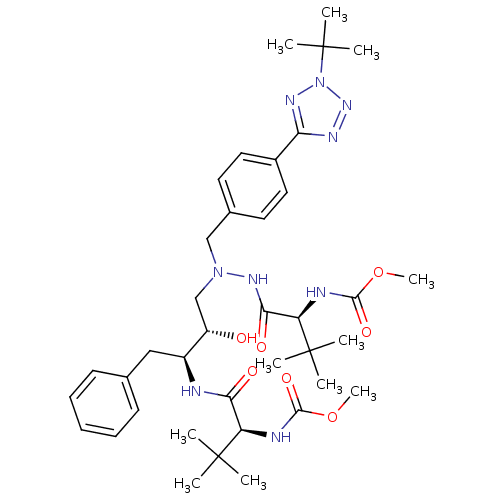 (((S)-1-{N'-[4-(2-tert-Butyl-2H-tetrazol-5-yl)-benz...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||