

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50066919 (2R,3R,4R,5R)-2,5-Bis-benzyloxy-3,4-dihydroxy-hexanedioic acid bis-[((S)-methylcarbamoyl-phenyl-methyl)-amide]::2,5-Bis-benzyloxy-3,4-dihydroxy-hexanedioic acid bis-[(methylcarbamoyl-phenyl-methyl)-amide]::CHEMBL124093
SMILES: CNC(=O)[C@@H](NC(=O)[C@H](OCc1ccccc1)[C@H](O)[C@@H](O)[C@@H](OCc1ccccc1)C(=O)N[C@H](C(=O)NC)c1ccccc1)c1ccccc1
InChI Key: InChIKey=SUXVPHKLQQOPHJ-XLTUSUNSSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM50066919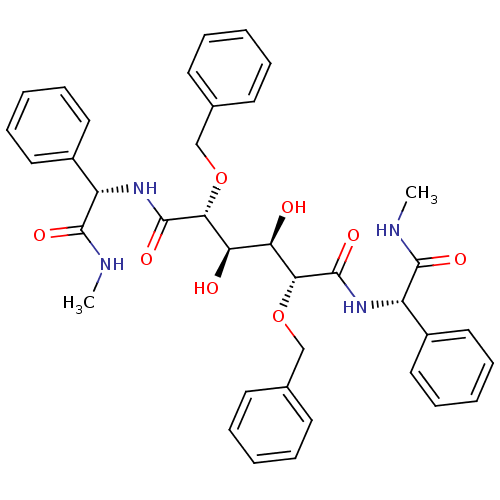 ((2R,3R,4R,5R)-2,5-Bis-benzyloxy-3,4-dihydroxy-hexa...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.30 | n/a | n/a | 27.4 | n/a | n/a | 2.05E+8 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM50066919 ((2R,3R,4R,5R)-2,5-Bis-benzyloxy-3,4-dihydroxy-hexa...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV-1 protease expressed in E. coli in sensitive fluorometric assay | J Med Chem 41: 3782-92 (1998) Article DOI: 10.1021/jm970777b BindingDB Entry DOI: 10.7270/Q2PK0F93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM50066919 ((2R,3R,4R,5R)-2,5-Bis-benzyloxy-3,4-dihydroxy-hexa...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||