

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50067498 (6aS,10aS)-3-(1,1-Dimethyl-heptyl)-9-hydroxymethyl-6,6-dimethyl-6a,7,10,10a-tetrahydro-6H-benzo[c]chromen-1-ol::(6aS,10aS)-9-(hydroxymethyl)-6,6-dimethyl-3-(2-methyloctan-2-yl)-6a,7,10,10a-tetrahydro-6H-benzo[c]chromen-1-ol::(6aS,7S)-3-(1,1-Dimethyl-heptyl)-9-hydroxymethyl-6,6-dimethyl-6a,7,10,10a-tetrahydro-6H-benzo[c]chromen-1-ol::CHEMBL334533::HU-210::HU-211
SMILES: CCCCCCC(C)(C)c1cc(O)c2[C@H]3CC(CO)=CC[C@@H]3C(C)(C)Oc2c1
InChI Key: InChIKey=SSQJFGMEZBFMNV-PMACEKPBSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50067498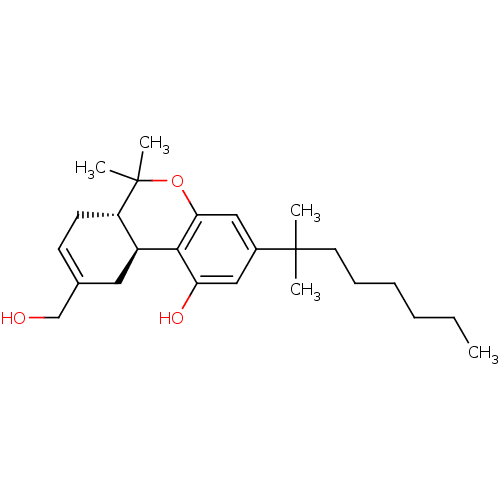 ((6aS,10aS)-3-(1,1-Dimethyl-heptyl)-9-hydroxymethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB2 receptor expressed in HEK cells | Bioorg Med Chem 15: 5406-16 (2007) Article DOI: 10.1016/j.bmc.2007.05.060 BindingDB Entry DOI: 10.7270/Q2T154G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50067498 ((6aS,10aS)-3-(1,1-Dimethyl-heptyl)-9-hydroxymethyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of [3H]-SR-141,716A binding to human CB1 receptor expressed in CHO cells | J Med Chem 48: 2509-17 (2005) Article DOI: 10.1021/jm049263k BindingDB Entry DOI: 10.7270/Q2Q23ZRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50067498 ((6aS,10aS)-3-(1,1-Dimethyl-heptyl)-9-hydroxymethyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor expressed in CHO cells | J Med Chem 48: 7486-90 (2005) Article DOI: 10.1021/jm0503906 BindingDB Entry DOI: 10.7270/Q22N5338 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50067498 ((6aS,10aS)-3-(1,1-Dimethyl-heptyl)-9-hydroxymethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB2 receptor expressed in COS cells | J Med Chem 48: 7343-50 (2005) Article DOI: 10.1021/jm0501533 BindingDB Entry DOI: 10.7270/Q2FX7B7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50067498 ((6aS,10aS)-3-(1,1-Dimethyl-heptyl)-9-hydroxymethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saint Louis University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 binding to Cannabinoid receptor 1 in rat brain membranes | J Med Chem 41: 4207-15 (1998) Article DOI: 10.1021/jm970239z BindingDB Entry DOI: 10.7270/Q2C24VK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50067498 ((6aS,10aS)-3-(1,1-Dimethyl-heptyl)-9-hydroxymethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from rat CB1 receptor by liquid scintillation spectrometry | Cell Chem Biol 56: 8224-56 (2013) Article DOI: 10.1021/jm4005626 BindingDB Entry DOI: 10.7270/Q2B859M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50067498 ((6aS,10aS)-3-(1,1-Dimethyl-heptyl)-9-hydroxymethyl...) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from rat CB2 receptor by liquid scintillation spectrometry | Cell Chem Biol 56: 8224-56 (2013) Article DOI: 10.1021/jm4005626 BindingDB Entry DOI: 10.7270/Q2B859M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50067498 ((6aS,10aS)-3-(1,1-Dimethyl-heptyl)-9-hydroxymethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.251 | n/a | n/a | n/a | n/a |
University of Kuopio Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells by [35]GTPgamma binding assay | J Med Chem 48: 7166-71 (2005) Article DOI: 10.1021/jm050565b BindingDB Entry DOI: 10.7270/Q2PC3552 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50067498 ((6aS,10aS)-3-(1,1-Dimethyl-heptyl)-9-hydroxymethyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Activity at human CB1 receptor by [35S]GTP-gamma-S binding stimulation assay | J Med Chem 48: 7486-90 (2005) Article DOI: 10.1021/jm0503906 BindingDB Entry DOI: 10.7270/Q22N5338 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||