

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50067527 3-(4-Carbamimidoyl-phenyl)-2-[2-(4-thiocarbamoyl-benzenesulfonylamino)-acetylamino]-propionic acid ethyl ester::CHEMBL137311
SMILES: CCOC(=O)C(Cc1ccc(cc1)C(N)=N)NC(=O)CNS(=O)(=O)c1ccc(cc1)C(N)=S
InChI Key: InChIKey=XUBBHZIMNINHAR-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thrombin (Bos taurus (Bovine)) | BDBM50067527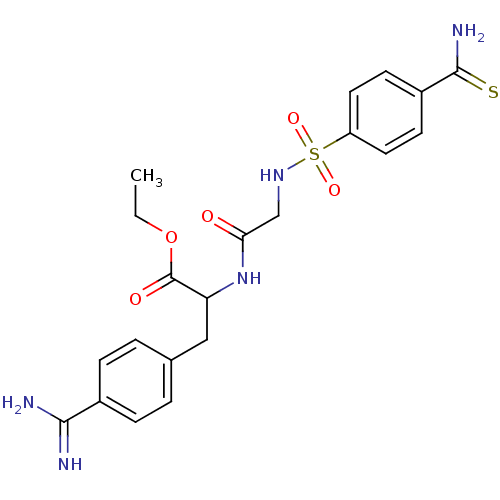 (3-(4-Carbamimidoyl-phenyl)-2-[2-(4-thiocarbamoyl-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibitory activity against Thrombin. | J Med Chem 41: 4240-50 (1998) Article DOI: 10.1021/jm980227t BindingDB Entry DOI: 10.7270/Q27D2T86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Bos taurus) | BDBM50067527 (3-(4-Carbamimidoyl-phenyl)-2-[2-(4-thiocarbamoyl-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X | J Med Chem 41: 4240-50 (1998) Article DOI: 10.1021/jm980227t BindingDB Entry DOI: 10.7270/Q27D2T86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50067527 (3-(4-Carbamimidoyl-phenyl)-2-[2-(4-thiocarbamoyl-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X | J Med Chem 41: 4240-50 (1998) Article DOI: 10.1021/jm980227t BindingDB Entry DOI: 10.7270/Q27D2T86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin II (Bos taurus) | BDBM50067527 (3-(4-Carbamimidoyl-phenyl)-2-[2-(4-thiocarbamoyl-b...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibitory activity against Trypsin. | J Med Chem 41: 4240-50 (1998) Article DOI: 10.1021/jm980227t BindingDB Entry DOI: 10.7270/Q27D2T86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50067527 (3-(4-Carbamimidoyl-phenyl)-2-[2-(4-thiocarbamoyl-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibitory activity against plasma kallikrein | J Med Chem 41: 4240-50 (1998) Article DOI: 10.1021/jm980227t BindingDB Entry DOI: 10.7270/Q27D2T86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50067527 (3-(4-Carbamimidoyl-phenyl)-2-[2-(4-thiocarbamoyl-b...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibitory activity against Plasmin. | J Med Chem 41: 4240-50 (1998) Article DOI: 10.1021/jm980227t BindingDB Entry DOI: 10.7270/Q27D2T86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50067527 (3-(4-Carbamimidoyl-phenyl)-2-[2-(4-thiocarbamoyl-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibitory activity against microPa. | J Med Chem 41: 4240-50 (1998) Article DOI: 10.1021/jm980227t BindingDB Entry DOI: 10.7270/Q27D2T86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K-dependent protein C (Homo sapiens (Human)) | BDBM50067527 (3-(4-Carbamimidoyl-phenyl)-2-[2-(4-thiocarbamoyl-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibitory activity against the anticoagulant Activated protein C | J Med Chem 41: 4240-50 (1998) Article DOI: 10.1021/jm980227t BindingDB Entry DOI: 10.7270/Q27D2T86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase (Homo sapiens (Human)) | BDBM50067527 (3-(4-Carbamimidoyl-phenyl)-2-[2-(4-thiocarbamoyl-b...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Biochemie Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Tryptase. | J Med Chem 41: 4240-50 (1998) Article DOI: 10.1021/jm980227t BindingDB Entry DOI: 10.7270/Q27D2T86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||