

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50068284 3-Benzyloxycarbonylamino-propionic acid 1,1,3-trioxo-1,3-dihydro-1lambda*6*-benzo[d]isothiazol-2-ylmethyl ester::CHEMBL344560
SMILES: O=C(CCNC(=O)OCc1ccccc1)OCN1C(=O)c2ccccc2S1(=O)=O
InChI Key: InChIKey=XGQXZQMZRZTGMF-UHFFFAOYSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trypsin II (Homo sapiens (Human)) | BDBM50068284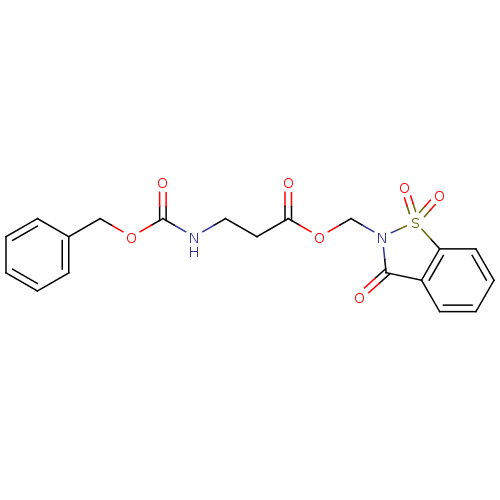 (3-Benzyloxycarbonylamino-propionic acid 1,1,3-trio...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity was measured against trypsin. | J Med Chem 41: 4854-60 (1998) Article DOI: 10.1021/jm9804580 BindingDB Entry DOI: 10.7270/Q21N8083 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50068284 (3-Benzyloxycarbonylamino-propionic acid 1,1,3-trio...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity was measured against plasmin. | J Med Chem 41: 4854-60 (1998) Article DOI: 10.1021/jm9804580 BindingDB Entry DOI: 10.7270/Q21N8083 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50068284 (3-Benzyloxycarbonylamino-propionic acid 1,1,3-trio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity was measured against urokinase. | J Med Chem 41: 4854-60 (1998) Article DOI: 10.1021/jm9804580 BindingDB Entry DOI: 10.7270/Q21N8083 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50068284 (3-Benzyloxycarbonylamino-propionic acid 1,1,3-trio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity was measured against Coagulation factor X | J Med Chem 41: 4854-60 (1998) Article DOI: 10.1021/jm9804580 BindingDB Entry DOI: 10.7270/Q21N8083 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukocyte elastase (Homo sapiens (Human)) | BDBM50068284 (3-Benzyloxycarbonylamino-propionic acid 1,1,3-trio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity was measured against human leukocyte elastase. | J Med Chem 41: 4854-60 (1998) Article DOI: 10.1021/jm9804580 BindingDB Entry DOI: 10.7270/Q21N8083 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50068284 (3-Benzyloxycarbonylamino-propionic acid 1,1,3-trio...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity was measured against thrombin. | J Med Chem 41: 4854-60 (1998) Article DOI: 10.1021/jm9804580 BindingDB Entry DOI: 10.7270/Q21N8083 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||