

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50071375 CHEMBL3410223::US10618900, No. CTEP
SMILES: Cc1nc(C#Cc2ccnc(Cl)c2)c(C)n1-c1ccc(OC(F)(F)F)cc1
InChI Key: InChIKey=GOHCTCOGYKAJLZ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50071375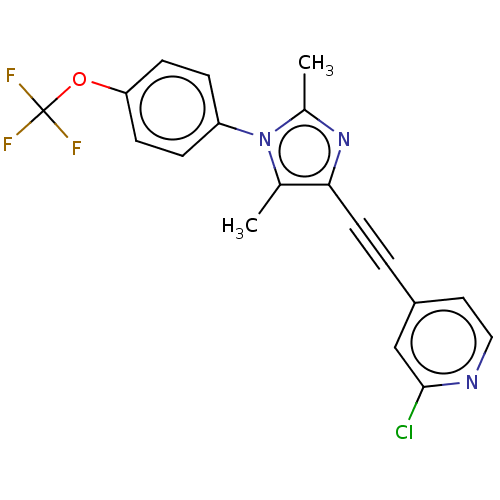 (CHEMBL3410223 | US10618900, No. CTEP) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceutical Research and Early Development Curated by ChEMBL | Assay Description Displacement of [3H]MPEP from mGlu5 receptor (unknown origin) expressed in HEK293 cells by competition binding assay | J Med Chem 58: 1358-71 (2015) Article DOI: 10.1021/jm501642c BindingDB Entry DOI: 10.7270/Q2J96825 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50071375 (CHEMBL3410223 | US10618900, No. CTEP) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceutical Research and Early Development Curated by ChEMBL | Assay Description Inhibition of human ERG channel | J Med Chem 58: 1358-71 (2015) Article DOI: 10.1021/jm501642c BindingDB Entry DOI: 10.7270/Q2J96825 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50071375 (CHEMBL3410223 | US10618900, No. CTEP) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.39E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica, Chinese Academy of Sciences US Patent | Assay Description Experimental material: HEK293/mGluR5 cell line, Fluo-8 calcium ion fluorescent dye, positive control MPEP, CTEPExperimental instrument: FLIPR Tetra r... | US Patent US10618900 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50071375 (CHEMBL3410223 | US10618900, No. CTEP) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceutical Research and Early Development Curated by ChEMBL | Assay Description Negative allosteric modulation of mGlu5 (unknown origin) expressed in HEK293 cells assessed as inhibition of L-AP4-induced calcium mobilization incub... | J Med Chem 58: 1358-71 (2015) Article DOI: 10.1021/jm501642c BindingDB Entry DOI: 10.7270/Q2J96825 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||