

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50071967 (R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)-N-((1S,2S)-1-{(S)-1-[2-((R)-1-benzylcarbamoyl-butylcarbamoyl)-pyrrolidine-1-carbonyl]-2-methyl-propylcarbamoyl}-2-methyl-butyl)-succinamic acid::CHEMBL2370189
SMILES: CCC[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(C)=O)[C@@H](C)CC)C(C)C)C(=O)NCc1ccccc1
InChI Key: InChIKey=SDJZZUWGQQRKKX-VOFONVMCSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pancreatic elastase (Sus scrofa (Pig)) | BDBM50071967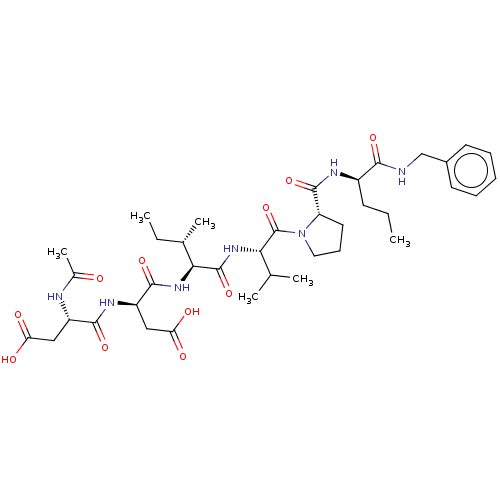 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of porcine pancreatic elastase (PPE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis C virus serine protease, NS3/NS4A (Hepatitis C virus) | BDBM50071967 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50071967 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of human liver Cathepsin B | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukocyte elastase (Homo sapiens (Human)) | BDBM50071967 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of human leucocyte elastase (HLE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||