 Found 8 hits for monomerid = 50072032
Found 8 hits for monomerid = 50072032 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072032
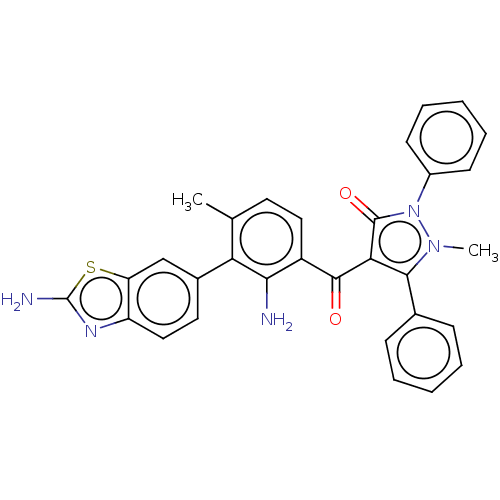
(CHEMBL3407858)Show SMILES Cc1ccc(C(=O)c2c(-c3ccccc3)n(C)n(-c3ccccc3)c2=O)c(N)c1-c1ccc2nc(N)sc2c1 Show InChI InChI=1S/C31H25N5O2S/c1-18-13-15-22(27(32)25(18)20-14-16-23-24(17-20)39-31(33)34-23)29(37)26-28(19-9-5-3-6-10-19)35(2)36(30(26)38)21-11-7-4-8-12-21/h3-17H,32H2,1-2H3,(H2,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GCN2 in amino acid starved human HT1080 cells assessed as inhibition of CHoP mRNA expression preincubated for 1 hr followed by 2 hrs in... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072032

(CHEMBL3407858)Show SMILES Cc1ccc(C(=O)c2c(-c3ccccc3)n(C)n(-c3ccccc3)c2=O)c(N)c1-c1ccc2nc(N)sc2c1 Show InChI InChI=1S/C31H25N5O2S/c1-18-13-15-22(27(32)25(18)20-14-16-23-24(17-20)39-31(33)34-23)29(37)26-28(19-9-5-3-6-10-19)35(2)36(30(26)38)21-11-7-4-8-12-21/h3-17H,32H2,1-2H3,(H2,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged PERK expressed in Escherichia coli using AviTag C-terminal, N-terminal His-tagged eIF2alpha (3 to 315) as s... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 4
(Homo sapiens (Human)) | BDBM50072032

(CHEMBL3407858)Show SMILES Cc1ccc(C(=O)c2c(-c3ccccc3)n(C)n(-c3ccccc3)c2=O)c(N)c1-c1ccc2nc(N)sc2c1 Show InChI InChI=1S/C31H25N5O2S/c1-18-13-15-22(27(32)25(18)20-14-16-23-24(17-20)39-31(33)34-23)29(37)26-28(19-9-5-3-6-10-19)35(2)36(30(26)38)21-11-7-4-8-12-21/h3-17H,32H2,1-2H3,(H2,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal His-tagged GCN2 expressed in Escherichia coli using AviTag C-terminal, N-terminal His-tagged eIF2alpha (3 to 315) as s... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072032

(CHEMBL3407858)Show SMILES Cc1ccc(C(=O)c2c(-c3ccccc3)n(C)n(-c3ccccc3)c2=O)c(N)c1-c1ccc2nc(N)sc2c1 Show InChI InChI=1S/C31H25N5O2S/c1-18-13-15-22(27(32)25(18)20-14-16-23-24(17-20)39-31(33)34-23)29(37)26-28(19-9-5-3-6-10-19)35(2)36(30(26)38)21-11-7-4-8-12-21/h3-17H,32H2,1-2H3,(H2,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GCN2 phosphorylation in human U2OS cells preincubated for 1 hr followed by drug wash out and incubated for 2 hrs by MSD assay |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072032

(CHEMBL3407858)Show SMILES Cc1ccc(C(=O)c2c(-c3ccccc3)n(C)n(-c3ccccc3)c2=O)c(N)c1-c1ccc2nc(N)sc2c1 Show InChI InChI=1S/C31H25N5O2S/c1-18-13-15-22(27(32)25(18)20-14-16-23-24(17-20)39-31(33)34-23)29(37)26-28(19-9-5-3-6-10-19)35(2)36(30(26)38)21-11-7-4-8-12-21/h3-17H,32H2,1-2H3,(H2,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of doxycycline-inducible T-REx-PERK-FLAG (unknown origin) autophosphorylation tranfected in human HT1080 cells after 1 hr by sandwich ELIS... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072032

(CHEMBL3407858)Show SMILES Cc1ccc(C(=O)c2c(-c3ccccc3)n(C)n(-c3ccccc3)c2=O)c(N)c1-c1ccc2nc(N)sc2c1 Show InChI InChI=1S/C31H25N5O2S/c1-18-13-15-22(27(32)25(18)20-14-16-23-24(17-20)39-31(33)34-23)29(37)26-28(19-9-5-3-6-10-19)35(2)36(30(26)38)21-11-7-4-8-12-21/h3-17H,32H2,1-2H3,(H2,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 210 | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PERK-mediated protein synthesis in human U2OS cells harboring thapsigargin-induced ER stress assessed as incorporation of L-azidohomoal... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 2-alpha kinase 3
(Homo sapiens (Human)) | BDBM50072032

(CHEMBL3407858)Show SMILES Cc1ccc(C(=O)c2c(-c3ccccc3)n(C)n(-c3ccccc3)c2=O)c(N)c1-c1ccc2nc(N)sc2c1 Show InChI InChI=1S/C31H25N5O2S/c1-18-13-15-22(27(32)25(18)20-14-16-23-24(17-20)39-31(33)34-23)29(37)26-28(19-9-5-3-6-10-19)35(2)36(30(26)38)21-11-7-4-8-12-21/h3-17H,32H2,1-2H3,(H2,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PERK in human HT1080 cells assessed as inhibition of thapsigargin-induced CHoP mRNA expression preincubated for 1 hr followed by thapsi... |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50072032

(CHEMBL3407858)Show SMILES Cc1ccc(C(=O)c2c(-c3ccccc3)n(C)n(-c3ccccc3)c2=O)c(N)c1-c1ccc2nc(N)sc2c1 Show InChI InChI=1S/C31H25N5O2S/c1-18-13-15-22(27(32)25(18)20-14-16-23-24(17-20)39-31(33)34-23)29(37)26-28(19-9-5-3-6-10-19)35(2)36(30(26)38)21-11-7-4-8-12-21/h3-17H,32H2,1-2H3,(H2,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant BRAF V600E kinase domain mutant (unknown origin) |
J Med Chem 58: 1426-41 (2015)
Article DOI: 10.1021/jm5017494
BindingDB Entry DOI: 10.7270/Q27W6DWN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data