

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50072819 4-[3-(4-Carbamoyl-2-oxy-furazan-3-ylmethoxy)-phenyl]-2,6-dimethyl-1,4-dihydro-pyridine-3,5-dicarboxylic acid dimethyl ester::CHEMBL345530
SMILES: COC(=O)C1=C(C)[N-]C(C)=C(C1c1cccc(OCc2c(no[n+]2[O-])C(N)=O)c1)C(=[OH+])OC
InChI Key: InChIKey=GLHGGTAWJJKJFX-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072819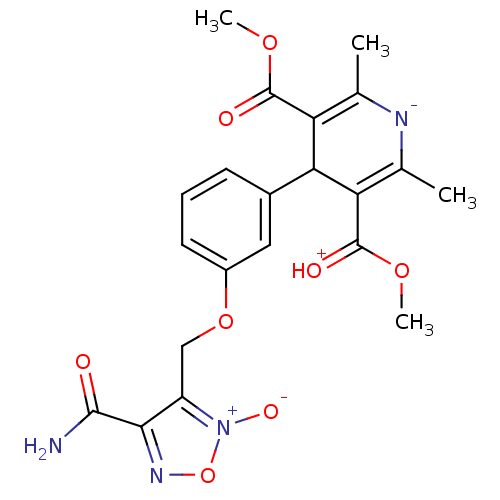 (4-[3-(4-Carbamoyl-2-oxy-furazan-3-ylmethoxy)-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Binding affinity for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1D (Rattus norvegicus) | BDBM50072819 (4-[3-(4-Carbamoyl-2-oxy-furazan-3-ylmethoxy)-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Torino Curated by ChEMBL | Assay Description Inhibitory concentration for L-type [Ca2+] channels was measured through displacement of [3H]nitrendipine on rat cortex homogenates. | J Med Chem 41: 5393-401 (1999) Article DOI: 10.1021/jm9803267 BindingDB Entry DOI: 10.7270/Q2WM1F30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||