

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50076642 2,2,2-Trifluoro-N-hydroxy-N-{3-[1-(4-methoxy-phenyl)-5-p-tolyl-1H-pyrazol-3-yl]-1-methyl-prop-2-ynyl}-acetamide::CHEMBL264200
SMILES: COc1ccc(cc1)-n1nc(cc1-c1ccc(C)cc1)C#CC(C)N(O)C(=O)C(F)(F)F
InChI Key: InChIKey=VYNUFKNGNAKQTM-UHFFFAOYSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase (cyclooxygenase) (Ovis aries (Sheep)) | BDBM50076642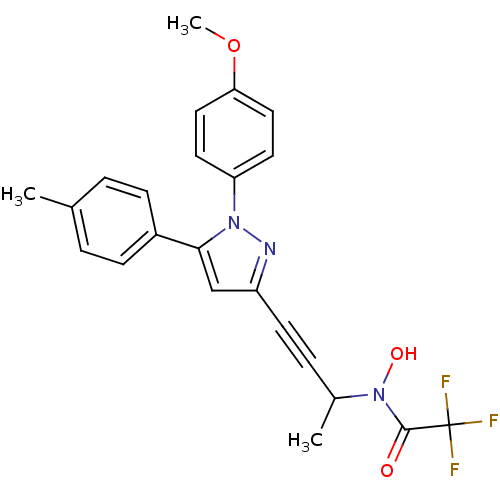 (2,2,2-Trifluoro-N-hydroxy-N-{3-[1-(4-methoxy-pheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cyclooxygenase using sheep seminal vesicle (SSV) enzyme (COX-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Rattus norvegicus) | BDBM50076642 (2,2,2-Trifluoro-N-hydroxy-N-{3-[1-(4-methoxy-pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against 5-lipoxygenase (5-LO) in intact rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (RAT) | BDBM50076642 (2,2,2-Trifluoro-N-hydroxy-N-{3-[1-(4-methoxy-pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cyclooxygenase (COX) using broken rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (RAT) | BDBM50076642 (2,2,2-Trifluoro-N-hydroxy-N-{3-[1-(4-methoxy-pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against cyclooxygenase (COX) in intact rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Rattus norvegicus) | BDBM50076642 (2,2,2-Trifluoro-N-hydroxy-N-{3-[1-(4-methoxy-pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against 5-lipoxygenase (5-LO) using broken rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||