

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50077360 3,10-Dihydroxy-13-(3-hydroxy-4-methoxy-phenyl)-2,11-dimethoxy-5-oxa-6b-aza-dibenzo[a,i]fluoren-6-one::CHEMBL301226
SMILES: COc1ccc(cc1O)-c1c2c(n3ccc4cc(O)c(OC)cc4c13)c(=O)oc1cc(O)c(OC)cc21
InChI Key: InChIKey=POCZBHBFCIWCCV-UHFFFAOYSA-N
Data: 7 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM50077360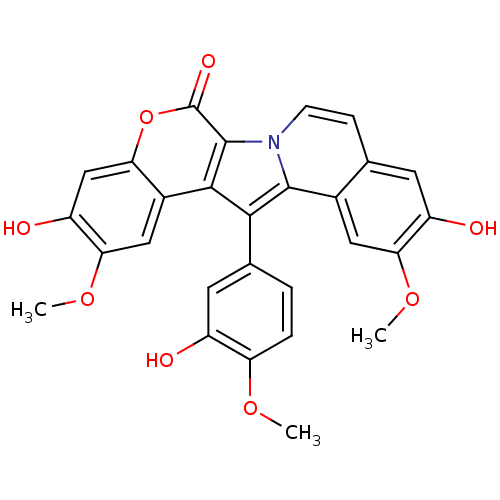 (3,10-Dihydroxy-13-(3-hydroxy-4-methoxy-phenyl)-2,1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of HIV-1 replication | J Med Chem 42: 1901-7 (1999) Article DOI: 10.1021/jm9806650 BindingDB Entry DOI: 10.7270/Q2PG1QWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM50077360 (3,10-Dihydroxy-13-(3-hydroxy-4-methoxy-phenyl)-2,1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibitory activity agaginst integrase as yield of terminal cleavage products | J Med Chem 42: 1901-7 (1999) Article DOI: 10.1021/jm9806650 BindingDB Entry DOI: 10.7270/Q2PG1QWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-Dependent Kinase 5 (CDK5) (Homo sapiens (Human)) | BDBM50077360 (3,10-Dihydroxy-13-(3-hydroxy-4-methoxy-phenyl)-2,1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of recombinant CDK5/p25 (unknown origin) expressed in Escherichia coli using [gamma-33P]ATP after 30 mins | J Med Chem 56: 7289-301 (2013) Article DOI: 10.1021/jm400719y BindingDB Entry DOI: 10.7270/Q2W37XQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50077360 (3,10-Dihydroxy-13-(3-hydroxy-4-methoxy-phenyl)-2,1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of recombinant EGFR (unknown origin) T790M/L858R double mutant preincubated for 30 mins followed by poly (Glu-Tyr) biotinylated peptide su... | Bioorg Med Chem 25: 6563-6580 (2017) Article DOI: 10.1016/j.bmc.2017.10.030 BindingDB Entry DOI: 10.7270/Q2RB7764 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50077360 (3,10-Dihydroxy-13-(3-hydroxy-4-methoxy-phenyl)-2,1...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) expressed in Escherichia coli using [gamma-33P]ATP after 30 mins | J Med Chem 56: 7289-301 (2013) Article DOI: 10.1021/jm400719y BindingDB Entry DOI: 10.7270/Q2W37XQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50077360 (3,10-Dihydroxy-13-(3-hydroxy-4-methoxy-phenyl)-2,1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of recombinant wild type EGFR (696 to C-terminal residues) (unknown origin) preincubated for 30 mins followed by poly (Glu-Tyr) biotinylat... | Bioorg Med Chem 25: 6563-6580 (2017) Article DOI: 10.1016/j.bmc.2017.10.030 BindingDB Entry DOI: 10.7270/Q2RB7764 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual-specificity tyrosine-phosphorylation regulated kinase 1A (Homo sapiens (Human)) | BDBM50077360 (3,10-Dihydroxy-13-(3-hydroxy-4-methoxy-phenyl)-2,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Inhibition of human recombinant DYRK1A expressed in Escherichia coli using [gamma-33P]ATP after 30 mins | J Med Chem 56: 7289-301 (2013) Article DOI: 10.1021/jm400719y BindingDB Entry DOI: 10.7270/Q2W37XQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||