

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: NC(=N)c1cccc(c1)C1=NOC(Cn2cnnn2)(C1)C(=O)Nc1ccc(cn1)-c1ccccc1S(N)(=O)=O
InChI Key: InChIKey=NWIZWPMWIKFVDY-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM50079240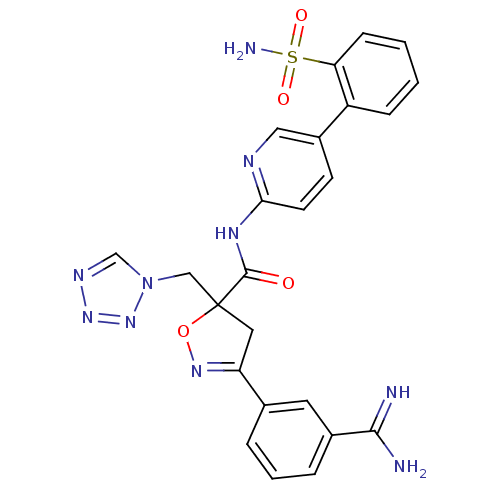 (3-(3-Carbamimidoyl-phenyl)-5-tetrazol-1-ylmethyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa using human purified enzyme. | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Oryctolagus cuniculus) | BDBM50079240 (3-(3-Carbamimidoyl-phenyl)-5-tetrazol-1-ylmethyl-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against Rabbit factor Xa was determined | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50079240 (3-(3-Carbamimidoyl-phenyl)-5-tetrazol-1-ylmethyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity towards human purified Coagulation factor Xa (FXa) | J Med Chem 42: 2760-73 (1999) Article DOI: 10.1021/jm980406a BindingDB Entry DOI: 10.7270/Q28W3CHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50079240 (3-(3-Carbamimidoyl-phenyl)-5-tetrazol-1-ylmethyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity towards human purified Coagulation factor Xa (FXa) | J Med Chem 42: 2760-73 (1999) Article DOI: 10.1021/jm980406a BindingDB Entry DOI: 10.7270/Q28W3CHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50079240 (3-(3-Carbamimidoyl-phenyl)-5-tetrazol-1-ylmethyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50079240 (3-(3-Carbamimidoyl-phenyl)-5-tetrazol-1-ylmethyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against trypsin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50079240 (3-(3-Carbamimidoyl-phenyl)-5-tetrazol-1-ylmethyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity towards trypsin obtained from human purified enzymes | J Med Chem 42: 2760-73 (1999) Article DOI: 10.1021/jm980406a BindingDB Entry DOI: 10.7270/Q28W3CHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50079240 (3-(3-Carbamimidoyl-phenyl)-5-tetrazol-1-ylmethyl-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin using human purified enzyme | Bioorg Med Chem Lett 13: 1023-8 (2003) BindingDB Entry DOI: 10.7270/Q21R6PWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50079240 (3-(3-Carbamimidoyl-phenyl)-5-tetrazol-1-ylmethyl-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Binding affinity towards thrombin obtained from human purified enzymes | J Med Chem 42: 2760-73 (1999) Article DOI: 10.1021/jm980406a BindingDB Entry DOI: 10.7270/Q28W3CHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||