 Found 12 hits for monomerid = 50079577
Found 12 hits for monomerid = 50079577 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
DNA polymerase beta
(Rattus norvegicus) | BDBM50079577
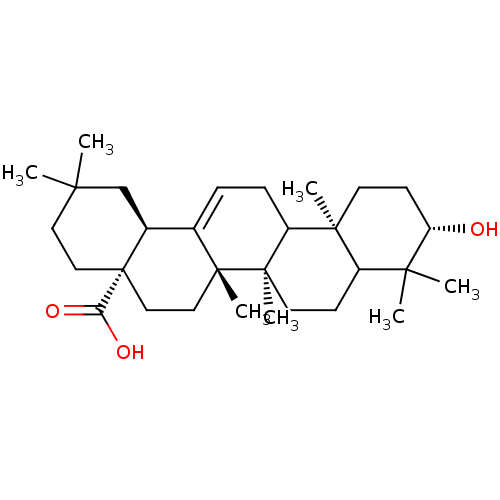
((4aS,6aS,6bR,10S,12aR,14bS)-10-Hydroxy-2,2,6a,6b,9...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CCC4[C@@]5(C)CC[C@H](O)C(C)(C)C5CC[C@@]34C)[C@@H]2C1)C(O)=O |c:10| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21?,22?,23-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of rat DNA polymerase beta in presence of BSA |
J Nat Prod 62: 1624-6 (2000)
BindingDB Entry DOI: 10.7270/Q2V69JBZ |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Rattus norvegicus) | BDBM50079577

((4aS,6aS,6bR,10S,12aR,14bS)-10-Hydroxy-2,2,6a,6b,9...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CCC4[C@@]5(C)CC[C@H](O)C(C)(C)C5CC[C@@]34C)[C@@H]2C1)C(O)=O |c:10| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21?,22?,23-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of rat DNA polymerase beta in absence of BSA |
J Nat Prod 62: 1624-6 (2000)
BindingDB Entry DOI: 10.7270/Q2V69JBZ |
More data for this
Ligand-Target Pair | |
Cyclooxygenase
(Homo sapiens (Human)) | BDBM50079577

((4aS,6aS,6bR,10S,12aR,14bS)-10-Hydroxy-2,2,6a,6b,9...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CCC4[C@@]5(C)CC[C@H](O)C(C)(C)C5CC[C@@]34C)[C@@H]2C1)C(O)=O |c:10| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21?,22?,23-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by scintillation proximity assay |
J Nat Prod 65: 1517-21 (2002)
BindingDB Entry DOI: 10.7270/Q2DV1JNG |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50079577

((4aS,6aS,6bR,10S,12aR,14bS)-10-Hydroxy-2,2,6a,6b,9...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CCC4[C@@]5(C)CC[C@H](O)C(C)(C)C5CC[C@@]34C)[C@@H]2C1)C(O)=O |c:10| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21?,22?,23-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase beta (unknown origin) lyase activity |
J Nat Prod 67: 964-7 (2004)
Article DOI: 10.1021/np030507y
BindingDB Entry DOI: 10.7270/Q2FQ9WDF |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1C
(Homo sapiens (Human)) | BDBM50079577

((4aS,6aS,6bR,10S,12aR,14bS)-10-Hydroxy-2,2,6a,6b,9...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CCC4[C@@]5(C)CC[C@H](O)C(C)(C)C5CC[C@@]34C)[C@@H]2C1)C(O)=O |c:10| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21?,22?,23-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant SHP1 |
Bioorg Med Chem 16: 8697-705 (2008)
Article DOI: 10.1016/j.bmc.2008.07.080
BindingDB Entry DOI: 10.7270/Q2HX1CH7 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM50079577

((4aS,6aS,6bR,10S,12aR,14bS)-10-Hydroxy-2,2,6a,6b,9...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CCC4[C@@]5(C)CC[C@H](O)C(C)(C)C5CC[C@@]34C)[C@@H]2C1)C(O)=O |c:10| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21?,22?,23-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College (Key Laboratory of Bioactive Substances and Resources Utilization of Chinese Herbal Medicine
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human PTP1B |
J Nat Prod 72: 1620-6 (2009)
Article DOI: 10.1021/np900305j
BindingDB Entry DOI: 10.7270/Q2HT2PFD |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase alpha
(Homo sapiens (Human)) | BDBM50079577

((4aS,6aS,6bR,10S,12aR,14bS)-10-Hydroxy-2,2,6a,6b,9...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CCC4[C@@]5(C)CC[C@H](O)C(C)(C)C5CC[C@@]34C)[C@@H]2C1)C(O)=O |c:10| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21?,22?,23-,27-,28+,29+,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTPalpha |
Bioorg Med Chem 16: 8697-705 (2008)
Article DOI: 10.1016/j.bmc.2008.07.080
BindingDB Entry DOI: 10.7270/Q2HX1CH7 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase epsilon (PTPε)
(Homo sapiens (Human)) | BDBM50079577

((4aS,6aS,6bR,10S,12aR,14bS)-10-Hydroxy-2,2,6a,6b,9...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CCC4[C@@]5(C)CC[C@H](O)C(C)(C)C5CC[C@@]34C)[C@@H]2C1)C(O)=O |c:10| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21?,22?,23-,27-,28+,29+,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTPepsilon |
Bioorg Med Chem 16: 8697-705 (2008)
Article DOI: 10.1016/j.bmc.2008.07.080
BindingDB Entry DOI: 10.7270/Q2HX1CH7 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM50079577

((4aS,6aS,6bR,10S,12aR,14bS)-10-Hydroxy-2,2,6a,6b,9...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CCC4[C@@]5(C)CC[C@H](O)C(C)(C)C5CC[C@@]34C)[C@@H]2C1)C(O)=O |c:10| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21?,22?,23-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) by pNPP assay |
Bioorg Med Chem 16: 8697-705 (2008)
Article DOI: 10.1016/j.bmc.2008.07.080
BindingDB Entry DOI: 10.7270/Q2HX1CH7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50079577

((4aS,6aS,6bR,10S,12aR,14bS)-10-Hydroxy-2,2,6a,6b,9...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CCC4[C@@]5(C)CC[C@H](O)C(C)(C)C5CC[C@@]34C)[C@@H]2C1)C(O)=O |c:10| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21?,22?,23-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP (unknown origin) by pNPP assay |
Bioorg Med Chem 16: 8697-705 (2008)
Article DOI: 10.1016/j.bmc.2008.07.080
BindingDB Entry DOI: 10.7270/Q2HX1CH7 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50079577

((4aS,6aS,6bR,10S,12aR,14bS)-10-Hydroxy-2,2,6a,6b,9...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CCC4[C@@]5(C)CC[C@H](O)C(C)(C)C5CC[C@@]34C)[C@@H]2C1)C(O)=O |c:10| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21?,22?,23-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase assessed as release of phosphate from glucose-1-phosphate after 25 mins |
J Nat Prod 72: 1414-8 (2009)
Article DOI: 10.1021/np9002367
BindingDB Entry DOI: 10.7270/Q2B8587T |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase F (LAR)
(Homo sapiens (Human)) | BDBM50079577

((4aS,6aS,6bR,10S,12aR,14bS)-10-Hydroxy-2,2,6a,6b,9...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CCC4[C@@]5(C)CC[C@H](O)C(C)(C)C5CC[C@@]34C)[C@@H]2C1)C(O)=O |c:10| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21?,22?,23-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant LAR |
Bioorg Med Chem 16: 8697-705 (2008)
Article DOI: 10.1016/j.bmc.2008.07.080
BindingDB Entry DOI: 10.7270/Q2HX1CH7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data