

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50079596 ((S)-1-{N'-[2-((S)-1-Benzyloxycarbonylamino-3-methyl-butyl)-thiazole-4-carbonyl]-hydrazinocarbonyl}-3-methyl-butyl)-carbamic acid benzyl ester::(1-{N'-[2-(1-Benzyloxycarbonylamino-3-methyl-butyl)-thiazole-4-carbonyl]-hydrazinocarbonyl}-3-methyl-butyl)-carbamic acid benzyl ester::CHEMBL57836::N-[2-[1-(N-BENZYLOXYCARBONYLAMINO)-3-METHYLBUTYL]THIAZOL-4-YLCARBONYL]-N'-(BENZYLOXYCARBONYL-L-LEUCINYL)HYDRAZIDE
SMILES: CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)c1csc(n1)[C@H](CC(C)C)NC(=O)OCc1ccccc1
InChI Key: InChIKey=FNMSKYFYFLZDOO-DQEYMECFSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Homo sapiens (Human)) | BDBM50079596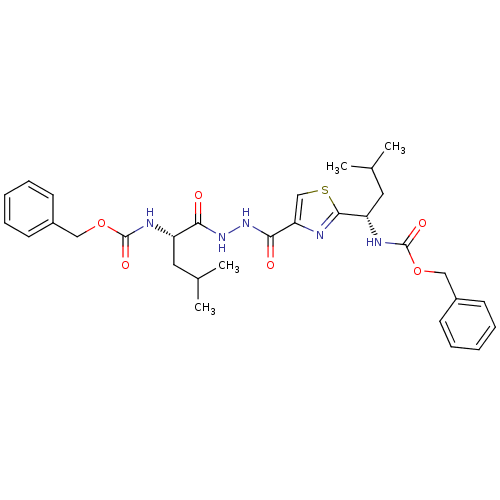 (((S)-1-{N'-[2-((S)-1-Benzyloxycarbonylamino-3-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH Curated by ChEMBL | Assay Description Inhibition of human cathepsin K | J Med Chem 56: 1478-90 (2013) Article DOI: 10.1021/jm3013932 BindingDB Entry DOI: 10.7270/Q2W09774 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50079596 (((S)-1-{N'-[2-((S)-1-Benzyloxycarbonylamino-3-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against human osteoclast cathepsin K | Bioorg Med Chem Lett 9: 1907-10 (1999) BindingDB Entry DOI: 10.7270/Q2M61JGJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||