

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50079856 CHEMBL108721::[2,6-Dibromo-4-(6-bromo-benzo[b]naphtho[2,3-d]thiophen-11-yl)-phenoxy]-acetic acid
SMILES: OC(=O)COc1c(Br)cc(cc1Br)-c1c2c(sc3ccccc23)c(Br)c2ccccc12
InChI Key: InChIKey=BOOMNQMMZXXWGF-UHFFFAOYSA-N
Data: 9 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor-type tyrosine-protein phosphatase beta (PTPβ) (Homo sapiens (Human)) | BDBM50079856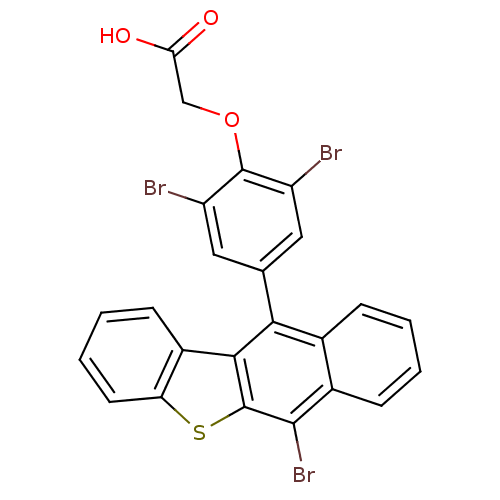 (CHEMBL108721 | [2,6-Dibromo-4-(6-bromo-benzo[b]nap...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description The compound was tested in vitro for the inhibitory activity against Protein-tyrosine phosphatase beta (SH-PTP1) (human PTPases.) | J Med Chem 42: 3199-202 (1999) Article DOI: 10.1021/jm990260v BindingDB Entry DOI: 10.7270/Q2DF6QCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM50079856 (CHEMBL108721 | [2,6-Dibromo-4-(6-bromo-benzo[b]nap...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description The compound was tested in vitro for the inhibitory activity against Protein-tyrosine phosphatase 1B (human PTPases.) | J Med Chem 42: 3199-202 (1999) Article DOI: 10.1021/jm990260v BindingDB Entry DOI: 10.7270/Q2DF6QCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM50079856 (CHEMBL108721 | [2,6-Dibromo-4-(6-bromo-benzo[b]nap...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 386 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description The compound was tested in vitro for the inhibitory activity against Protein-tyrosine phosphatase 1B (human PTPases.) | J Med Chem 42: 3199-202 (1999) Article DOI: 10.1021/jm990260v BindingDB Entry DOI: 10.7270/Q2DF6QCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase alpha (Homo sapiens (Human)) | BDBM50079856 (CHEMBL108721 | [2,6-Dibromo-4-(6-bromo-benzo[b]nap...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description The compound was tested in vitro for the inhibitory activity against Protein-tyrosine phosphatase 1 alpha (LRP) (human PTPases.) | J Med Chem 42: 3199-202 (1999) Article DOI: 10.1021/jm990260v BindingDB Entry DOI: 10.7270/Q2DF6QCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 12 (PTP-PEST) (Homo sapiens (Human)) | BDBM50079856 (CHEMBL108721 | [2,6-Dibromo-4-(6-bromo-benzo[b]nap...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description The compound was tested in vitro for the inhibitory activity against Protein tyrosine phosphatase PEST (SH-PTP1) (human PTPases.) | J Med Chem 42: 3199-202 (1999) Article DOI: 10.1021/jm990260v BindingDB Entry DOI: 10.7270/Q2DF6QCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1C (Homo sapiens (Human)) | BDBM50079856 (CHEMBL108721 | [2,6-Dibromo-4-(6-bromo-benzo[b]nap...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description The compound was tested in vitro for the inhibitory activity against Protein-tyrosine phosphatase 1C (SH-PTP1) (human PTPases.) | J Med Chem 42: 3199-202 (1999) Article DOI: 10.1021/jm990260v BindingDB Entry DOI: 10.7270/Q2DF6QCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukocyte common antigen (Homo sapiens (Human)) | BDBM50079856 (CHEMBL108721 | [2,6-Dibromo-4-(6-bromo-benzo[b]nap...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description The compound was tested in vitro for the inhibitory activity against CD45 antigen (human PTPases.) | J Med Chem 42: 3199-202 (1999) Article DOI: 10.1021/jm990260v BindingDB Entry DOI: 10.7270/Q2DF6QCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase (VHR) (Homo sapiens (Human)) | BDBM50079856 (CHEMBL108721 | [2,6-Dibromo-4-(6-bromo-benzo[b]nap...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description The compound was tested in vitro for the inhibitory activity against Dual specificity protein phosphatase 3 (SH-PTP1) (human PTPases.) | J Med Chem 42: 3199-202 (1999) Article DOI: 10.1021/jm990260v BindingDB Entry DOI: 10.7270/Q2DF6QCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase F (LAR) (Homo sapiens (Human)) | BDBM50079856 (CHEMBL108721 | [2,6-Dibromo-4-(6-bromo-benzo[b]nap...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description The compound was tested in vitro for the inhibitory activity against LAR (human Protein-tyrosine phosphatase F) | J Med Chem 42: 3199-202 (1999) Article DOI: 10.1021/jm990260v BindingDB Entry DOI: 10.7270/Q2DF6QCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||