

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC(C)C[C@@H](CO)Nc1nc(nc2[nH]c(C)c(C)c12)-c1ccccc1
InChI Key: InChIKey=GSMWUWXMHHXWOW-INIZCTEOSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50080286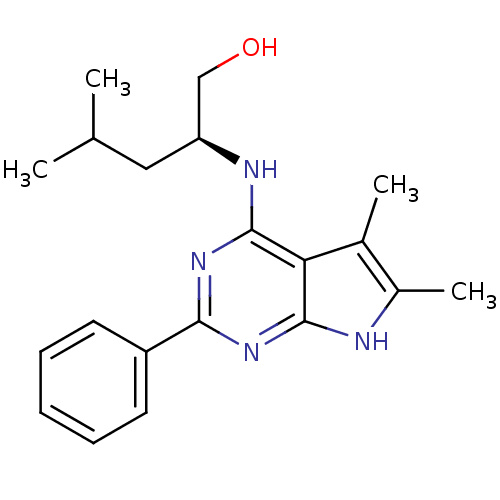 ((S)-2-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 951 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Inhibitory activity against binding of the [3H]-DPCPX to human A1 receptor (hA1) radioligands using competition binding assay | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50080286 ((S)-2-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Inhibitory activity against binding of [3H]-DPCPX to human A1 receptor (hA1) using competition binding assay | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50080286 ((S)-2-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Inhibitory activity against membranes from yeast cells transformed with human A1 receptor (hA1) | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50080286 ((S)-2-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Inhibitory activity against membranes from yeast cells transformed with human A1 receptor (hA1) | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50080286 ((S)-2-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Inhibitory activity against membranes from HEK293 cells stably expressing the human A2a receptor (hA2a) | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50080286 ((S)-2-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity towards human A2 receptor (hA2) was measured through displacement of [3H]-CGS-21,680 using yeast cell membranes | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||