

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50080559 CHEMBL3126433::US9617250, Example 8
SMILES: CCc1cc(cc(C)c1OC[C@@H](O)CNC(=O)CO)-c1noc(n1)-c1ccnc(c1)C1CCCC1
InChI Key: InChIKey=XRJPXSNXMMSPIZ-NRFANRHFSA-N
Data: 6 EC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50080559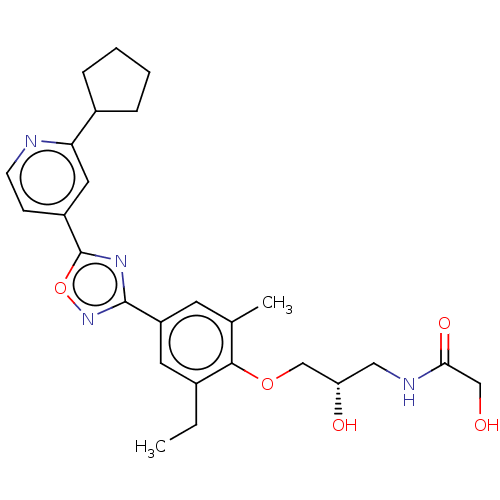 (CHEMBL3126433 | US9617250, Example 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Dart Neuroscience LLC Curated by ChEMBL | Assay Description Agonist activity at human recombinant S1P1 receptor expressed in CHO cells incubated for 30 mins prior to 35SGTP-gammaS addition measured after 1 hr ... | ACS Med Chem Lett 6: 102-3 (2015) Article DOI: 10.1021/ml500484v BindingDB Entry DOI: 10.7270/Q2S1847X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50080559 (CHEMBL3126433 | US9617250, Example 8) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 736 | n/a | n/a | n/a | n/a |
Dart Neuroscience LLC Curated by ChEMBL | Assay Description Agonist activity at human recombinant S1P3 receptor expressed in CHO cells incubated for 30 mins prior to 35SGTP-gammaS addition measured after 1 hr ... | ACS Med Chem Lett 6: 102-3 (2015) Article DOI: 10.1021/ml500484v BindingDB Entry DOI: 10.7270/Q2S1847X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50080559 (CHEMBL3126433 | US9617250, Example 8) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 736 | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Agonist activity at human recombinant S1P3 receptor expressed in CHO cells incubated for 30 mins prior to [35S]-GTPgammaS addition measured after 1 h... | J Med Chem 57: 110-30 (2014) Article DOI: 10.1021/jm4014696 BindingDB Entry DOI: 10.7270/Q2KK9FSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50080559 (CHEMBL3126433 | US9617250, Example 8) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 736 | n/a | n/a | n/a | n/a |
ACTELION PHARMACEUTICALS LTD. US Patent | Assay Description GTPγS binding assays are performed in 96 well microtiter plates (Nunc, 442587) in a final volume of 200 μl, using membrane preparations of ... | US Patent US9617250 (2017) BindingDB Entry DOI: 10.7270/Q2P55QKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50080559 (CHEMBL3126433 | US9617250, Example 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Agonist activity at human recombinant S1P1 receptor expressed in CHO cells incubated for 30 mins prior to [35S]-GTPgammaS addition measured after 1 h... | J Med Chem 57: 110-30 (2014) Article DOI: 10.1021/jm4014696 BindingDB Entry DOI: 10.7270/Q2KK9FSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50080559 (CHEMBL3126433 | US9617250, Example 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
ACTELION PHARMACEUTICALS LTD. US Patent | Assay Description GTPγS binding assays are performed in 96 well microtiter plates (Nunc, 442587) in a final volume of 200 μl, using membrane preparations of ... | US Patent US9617250 (2017) BindingDB Entry DOI: 10.7270/Q2P55QKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||