

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: [NH3+]Cc1ccc(F)c([O-])c1F
InChI Key: InChIKey=ARLSXSJLCYMMDM-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4-aminobutyrate aminotransferase, mitochondrial (Sus scrofa) | BDBM50082127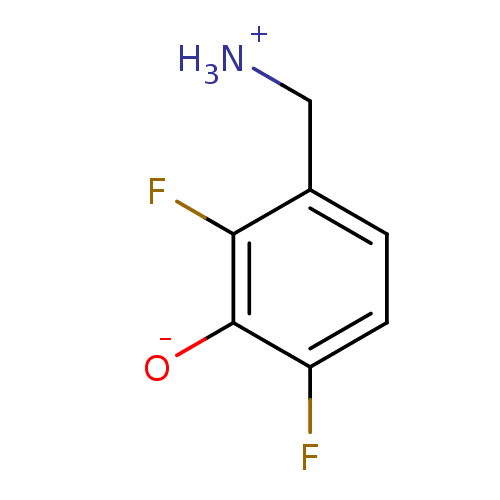 (3-(ammoniomethyl)-2,6-difluorobenzenolate | 3-Amin...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description The compound was tested for the inhibition of Gamma-amino-N-butyrate transaminase from pig brain | J Med Chem 42: 329-32 (1999) Article DOI: 10.1021/jm980435l BindingDB Entry DOI: 10.7270/Q25B01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Sus scrofa) | BDBM50082127 (3-(ammoniomethyl)-2,6-difluorobenzenolate | 3-Amin...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Competitive inhibition of pig brain GABA aminotransferase by Dixon/Cornish-Bowden plot analysis in presence of GABA | J Med Chem 58: 8315-59 (2015) BindingDB Entry DOI: 10.7270/Q22J6DPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50082127 (3-(ammoniomethyl)-2,6-difluorobenzenolate | 3-Amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Competitive inhibition of GABA aminotransferase (unknown origin) | J Med Chem 61: 5822-5880 (2018) Article DOI: 10.1021/acs.jmedchem.7b01788 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-aminobutyrate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50082127 (3-(ammoniomethyl)-2,6-difluorobenzenolate | 3-Amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Competitive inhibition of GABA aminotransferase | J Med Chem 54: 2529-91 (2011) Article DOI: 10.1021/jm1013693 BindingDB Entry DOI: 10.7270/Q24M95PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid type B receptor subunit 1/2 (Rattus norvegicus (Rat)) | BDBM50082127 (3-(ammoniomethyl)-2,6-difluorobenzenolate | 3-Amin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney Curated by ChEMBL | Assay Description Displacement of [3H]GABA from Gamma-aminobutyric acid type B receptor in rat brain membranes | Bioorg Med Chem Lett 9: 3093-8 (1999) BindingDB Entry DOI: 10.7270/Q24J0FMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||