

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50082841 3-{3-[4-(2-Carbamoyl-phenyl)-piperazin-1-yl]-propylcarbamoyl}-4-(3,4-difluoro-phenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydro-pyrimidine-5-carboxylic acid methyl ester::CHEMBL145394
SMILES: COC(=O)C1=C(C)NC(=O)N(C1c1ccc(F)c(F)c1)C(=O)NCCCN1CCN(CC1)c1ccccc1C(N)=O
InChI Key: InChIKey=DMMNMIJPRNMLBQ-UHFFFAOYSA-N
Data: 7 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082841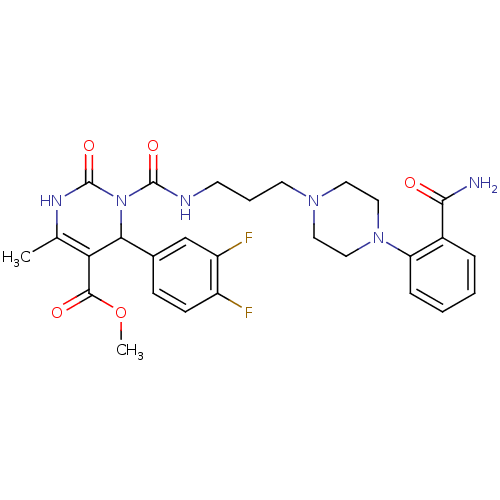 (3-{3-[4-(2-Carbamoyl-phenyl)-piperazin-1-yl]-propy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082841 (3-{3-[4-(2-Carbamoyl-phenyl)-piperazin-1-yl]-propy...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adrenergic alpha1B (Homo sapiens (Human)) | BDBM50082841 (3-{3-[4-(2-Carbamoyl-phenyl)-piperazin-1-yl]-propy...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1B adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adrenergic alpha1B (Homo sapiens (Human)) | BDBM50082841 (3-{3-[4-(2-Carbamoyl-phenyl)-piperazin-1-yl]-propy...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1B adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50082841 (3-{3-[4-(2-Carbamoyl-phenyl)-piperazin-1-yl]-propy...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1D adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50082841 (3-{3-[4-(2-Carbamoyl-phenyl)-piperazin-1-yl]-propy...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1D adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50082841 (3-{3-[4-(2-Carbamoyl-phenyl)-piperazin-1-yl]-propy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affiity of the compound against opioid receptor using [3H]-DAMGO as radioligand. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||