

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50083974 2-Amino-N-[(R)-2-((R)-3a-benzyl-2-methyl-3-oxo-2,3,3a,4,6,7-hexahydro-pyrazolo[4,3-c]pyridin-5-yl)-1-benzyloxymethyl-2-oxo-ethyl]-2-methyl-propionamide::CHEMBL113313::CP-424391-18
SMILES: CN1N=C2CCN(C[C@@]2(Cc2ccccc2)C1=O)C(=O)[C@@H](COCc1ccccc1)NC(=O)C(C)(C)N
InChI Key: InChIKey=KVLLHLWBPNCVNR-SKCUWOTOSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ghrelin receptor (Homo sapiens (Human)) | BDBM50083974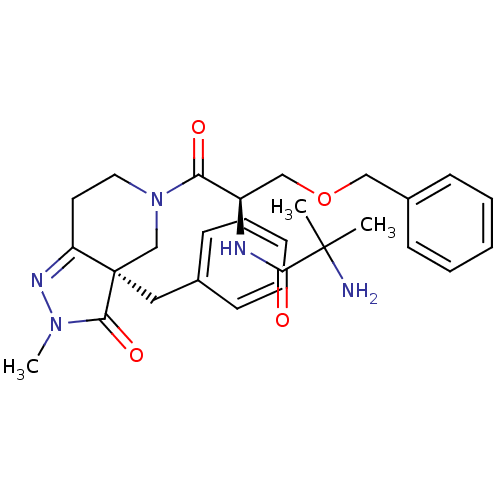 (2-Amino-N-[(R)-2-((R)-3a-benzyl-2-methyl-3-oxo-2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... | Bioorg Med Chem Lett 12: 3279-82 (2002) BindingDB Entry DOI: 10.7270/Q23F4NZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ghrelin receptor (Homo sapiens (Human)) | BDBM50083974 (2-Amino-N-[(R)-2-((R)-3a-benzyl-2-methyl-3-oxo-2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.912 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description In vitro inhibition of dihydrofolate reductase enzymes in Neisseria gonorrhoeae | J Med Chem 61: 5974-5987 (2018) Article DOI: 10.1021/acs.jmedchem.8b00322 BindingDB Entry DOI: 10.7270/Q2WD433X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Rattus norvegicus) | BDBM50083974 (2-Amino-N-[(R)-2-((R)-3a-benzyl-2-methyl-3-oxo-2,3...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ghrelin receptor (Homo sapiens (Human)) | BDBM50083974 (2-Amino-N-[(R)-2-((R)-3a-benzyl-2-methyl-3-oxo-2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Ability to inhibit HMG-CoA reductase (HMGR) by cholesterol synthesis inhibition screen (CSI) in rats | J Med Chem 61: 5974-5987 (2018) Article DOI: 10.1021/acs.jmedchem.8b00322 BindingDB Entry DOI: 10.7270/Q2WD433X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ghrelin receptor (Homo sapiens (Human)) | BDBM50083974 (2-Amino-N-[(R)-2-((R)-3a-benzyl-2-methyl-3-oxo-2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of human [125I]-ghrelin from GHS-R1a (unknown origin) expressed in HEK293 cell membranes after 1 hr by radioligand binding assay | J Med Chem 61: 5974-5987 (2018) Article DOI: 10.1021/acs.jmedchem.8b00322 BindingDB Entry DOI: 10.7270/Q2WD433X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ghrelin receptor (Homo sapiens (Human)) | BDBM50083974 (2-Amino-N-[(R)-2-((R)-3a-benzyl-2-methyl-3-oxo-2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for human growth hormone GH secretagogue (hGHsr) receptor | Bioorg Med Chem Lett 10: 5-8 (2000) BindingDB Entry DOI: 10.7270/Q2DV1KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ghrelin receptor (Homo sapiens (Human)) | BDBM50083974 (2-Amino-N-[(R)-2-((R)-3a-benzyl-2-methyl-3-oxo-2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description In vitro inhibition of dihydrofolate reductase enzymes in Neisseria gonorrhoeae | J Med Chem 61: 5974-5987 (2018) Article DOI: 10.1021/acs.jmedchem.8b00322 BindingDB Entry DOI: 10.7270/Q2WD433X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||