

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50084978 3,5,7-trihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-one::CHEMBL40919::Kaempferide::Kaempferol 4'-methyl ether
SMILES: COc1ccc(cc1)-c1oc2cc(O)cc(O)c2c(=O)c1O
InChI Key: InChIKey=SQFSKOYWJBQGKQ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50084978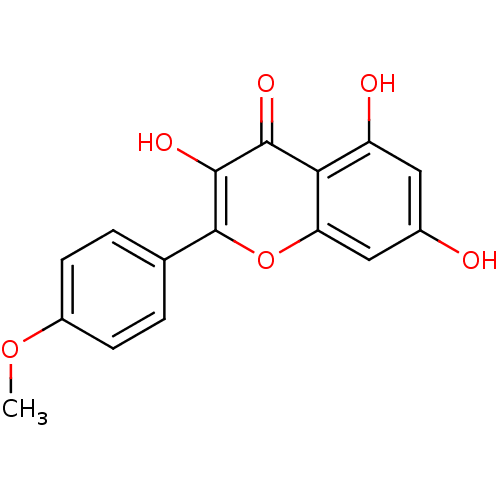 (3,5,7-trihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.29E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETD7 (Homo sapiens (Human)) | BDBM50084978 (3,5,7-trihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human SET7 overexpressed in Escherichia coli BL21 (DE3) cells preincubated for 15 mins followed by addition of SAM as substrate and bio... | Bioorg Med Chem 28: (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-glycoprotein (P-gp) (Mus musculus (Mouse)) | BDBM50084978 (3,5,7-trihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a |
Département de Pharmacochimie Moléculaire UMR-CNRS 5063 Curated by ChEMBL | Assay Description Binding affinity of the compound to nucleotide-binding domain (NBD2) of P-Glycoprotein | Bioorg Med Chem Lett 11: 75-7 (2001) BindingDB Entry DOI: 10.7270/Q2T43SC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Agaricus bisporus (Common mushroom)) | BDBM50084978 (3,5,7-trihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase | Bioorg Med Chem 17: 6048-53 (2009) Article DOI: 10.1016/j.bmc.2009.06.057 BindingDB Entry DOI: 10.7270/Q20G3K7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM50084978 (3,5,7-trihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-...) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 509 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 by EROD assay | Bioorg Med Chem 18: 6310-5 (2010) Article DOI: 10.1016/j.bmc.2010.07.020 BindingDB Entry DOI: 10.7270/Q2GB248D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50084978 (3,5,7-trihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Inhibition of human CYP1A1 by EROD assay | Bioorg Med Chem 18: 6310-5 (2010) Article DOI: 10.1016/j.bmc.2010.07.020 BindingDB Entry DOI: 10.7270/Q2GB248D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50084978 (3,5,7-trihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Inhibition of human CYP1B1 by EROD assay | Bioorg Med Chem 18: 6310-5 (2010) Article DOI: 10.1016/j.bmc.2010.07.020 BindingDB Entry DOI: 10.7270/Q2GB248D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-glycoprotein (P-gp) (Mus musculus (Mouse)) | BDBM50084978 (3,5,7-trihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard-Lyon 1 Curated by ChEMBL | Assay Description Compound was tested for the binding affinity towards recombinant NBD2 C-terminal cytotoxic nucleotide-binding domain of mouse P-Glycoprotein | Bioorg Med Chem Lett 10: 157-60 (2000) BindingDB Entry DOI: 10.7270/Q2DR2W1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50084978 (3,5,7-trihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of CYP1A1 | Bioorg Med Chem 15: 5047-60 (2007) Article DOI: 10.1016/j.bmc.2007.05.046 BindingDB Entry DOI: 10.7270/Q20R9Q7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM50084978 (3,5,7-trihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-...) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of CYP1A2 | Bioorg Med Chem 15: 5047-60 (2007) Article DOI: 10.1016/j.bmc.2007.05.046 BindingDB Entry DOI: 10.7270/Q20R9Q7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50084978 (3,5,7-trihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of CYP1B1 | Bioorg Med Chem 15: 5047-60 (2007) Article DOI: 10.1016/j.bmc.2007.05.046 BindingDB Entry DOI: 10.7270/Q20R9Q7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-glycoprotein 1 (Homo sapiens (Human)) | BDBM50084978 (3,5,7-trihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 4.47E+3 | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Binding affinity to ABCB1 nucleotide binding domain 2 | Eur J Med Chem 46: 4078-88 (2011) Article DOI: 10.1016/j.ejmech.2011.06.008 BindingDB Entry DOI: 10.7270/Q2FJ2J3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA Gyrase (Escherichia coli (strain K12)) | BDBM50084978 (3,5,7-trihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | n/a | n/a | >1.66E+6 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro antibacterial activity was determined as inhibitory concentration causing 50% DNA-gyrase supercoiling inhibition (SCI) | Citation and Details BindingDB Entry DOI: 10.7270/Q2FX7CM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase (flavin-containing) A (Homo sapiens (Human)) | BDBM50084978 (3,5,7-trihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunchon National University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A expressed in baculovirus infected BTI insect cells using kynuramine as substrate after 20 mins by spectrophotom... | Bioorg Med Chem Lett 29: 839-843 (2019) Article DOI: 10.1016/j.bmcl.2019.01.016 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50084978 (3,5,7-trihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.25E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Eberhard Karls University of Tuebingen | Assay Description The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n... | Chembiochem 11: 2579-88 (2010) Article DOI: 10.1002/cbic.201000487 BindingDB Entry DOI: 10.7270/Q21C1VDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||