

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50085551 1,8-Dihydroxy-3-hydroxymethyl-anthraquinone::1,8-dihydroxy-3-(hydroxymethyl)anthracene-9,10-dione::CHEMBL40275::aloe emodin::aloe-emodin::cid_10207
SMILES: OCc1cc(O)c2C(=O)c3c(O)cccc3C(=O)c2c1
InChI Key: InChIKey=YDQWDHRMZQUTBA-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Purinergic receptor P2Y12 (Homo sapiens (Human)) | BDBM50085551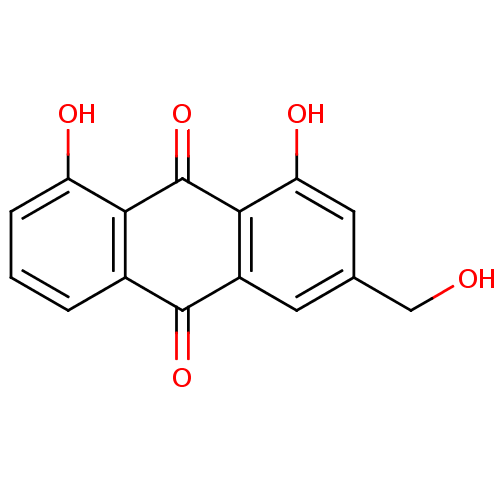 (1,8-Dihydroxy-3-hydroxymethyl-anthraquinone | 1,8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]PSB0413 from human platelet P2Y12 receptor | J Med Chem 52: 3784-93 (2009) Article DOI: 10.1021/jm9003297 BindingDB Entry DOI: 10.7270/Q2NK3FZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| EBifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50085551 (1,8-Dihydroxy-3-hydroxymethyl-anthraquinone | 1,8-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 5.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Non-competitive inhibition of sEH (unknown origin) using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde measured during 30 mi... | Bioorg Med Chem 23: 6659-65 (2015) BindingDB Entry DOI: 10.7270/Q22F7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Homo sapiens (Human)) | BDBM50085551 (1,8-Dihydroxy-3-hydroxymethyl-anthraquinone | 1,8-...) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2H130G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpi (Rattus norvegicus (Rat)) | BDBM50085551 (1,8-Dihydroxy-3-hydroxymethyl-anthraquinone | 1,8-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | 2.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2DB809V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50085551 (1,8-Dihydroxy-3-hydroxymethyl-anthraquinone | 1,8-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) by Ellman's method | Bioorg Med Chem Lett 24: 5385-9 (2015) Article DOI: 10.1016/j.bmcl.2014.10.049 BindingDB Entry DOI: 10.7270/Q2028T5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3C-like proteinase (3CL-PRO) (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50085551 (1,8-Dihydroxy-3-hydroxymethyl-anthraquinone | 1,8-...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.32E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of recombinant SARS coronavirus 3C-like protease trans-cleavage activity by ELISA | Bioorg Med Chem 21: 3730-7 (2013) Article DOI: 10.1016/j.bmc.2013.04.026 BindingDB Entry DOI: 10.7270/Q24J0J2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, cytosolic 1 (Rattus norvegicus) | BDBM50085551 (1,8-Dihydroxy-3-hydroxymethyl-anthraquinone | 1,8-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat cytosolic aldehyde dehydrogenase | J Nat Prod 60: 1180-1182 (1997) Article DOI: 10.1021/np9703104 BindingDB Entry DOI: 10.7270/Q2DR2VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase 1, cytoplasmic (Rattus norvegicus) | BDBM50085551 (1,8-Dihydroxy-3-hydroxymethyl-anthraquinone | 1,8-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of rat liver cytosolic TrxR1 by spectrophotometry | Bioorg Med Chem 19: 631-41 (2011) Article DOI: 10.1016/j.bmc.2010.10.045 BindingDB Entry DOI: 10.7270/Q25D8S4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase 2, mitochondrial (Rattus norvegicus) | BDBM50085551 (1,8-Dihydroxy-3-hydroxymethyl-anthraquinone | 1,8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of rat liver mitochondrial TrxR2 by spectrophotometry | Bioorg Med Chem 19: 631-41 (2011) Article DOI: 10.1016/j.bmc.2010.10.045 BindingDB Entry DOI: 10.7270/Q25D8S4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase placental-like (Homo sapiens (Human)) | BDBM50085551 (1,8-Dihydroxy-3-hydroxymethyl-anthraquinone | 1,8-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2NS0SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal alkaline phosphatase (Homo sapiens (Human)) | BDBM50085551 (1,8-Dihydroxy-3-hydroxymethyl-anthraquinone | 1,8-...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego, CA... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2X63KDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| EBifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50085551 (1,8-Dihydroxy-3-hydroxymethyl-anthraquinone | 1,8-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of sEH (unknown origin) using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde measured during 1 hr by fluorescence ... | Bioorg Med Chem 23: 6659-65 (2015) BindingDB Entry DOI: 10.7270/Q22F7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||